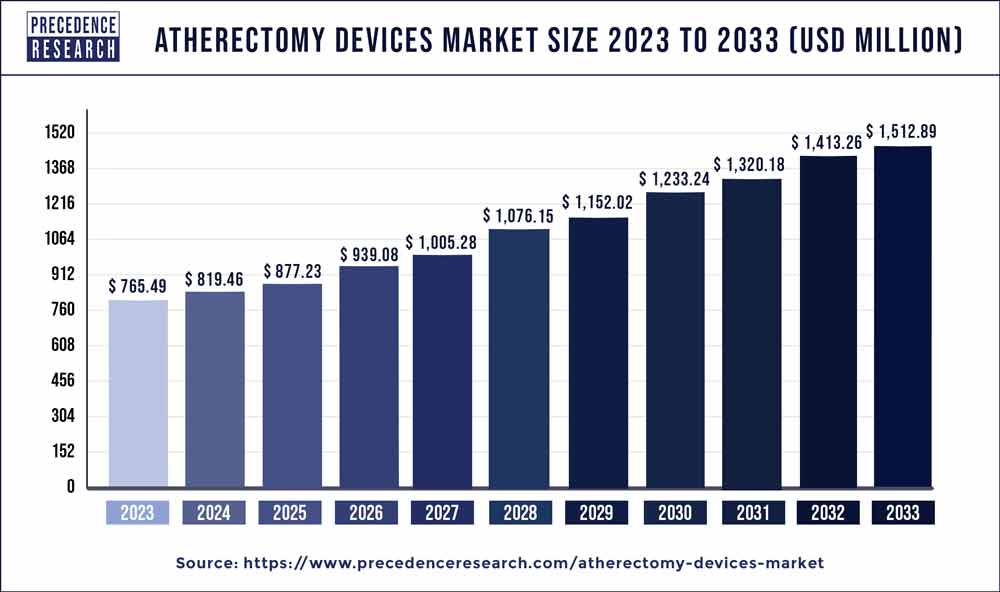
The global atherectomy devices market size reached USD 765.49 million in 2023 and is projected to grow around USD 1,512.89 million by 2033, growing at a CAGR of 7.05% from 2024 to 2033.
Key Points
- North America led the market with the biggest market share of 45% in 2023.
- Asia Pacific is projected to expand at the fastest rate during the forecast period of 2024-2033.
- By device type, the drug-coated balloons (DCBs) segment held the largest share of the market in 2023.
- By device type, the intravascular ultrasound (IVUS) catheter segment is expected to show the fastest growth.
- By application type, the peripheral artery disease segment held the dominating share of the market in 2023.
- By application type, the coronary artery disease segment represents another highly influential segment for the forecast period.
- By end-user, the cardiac catheterization labs segment held the dominating share of the market in 2023.
- By end-user, the interventional radiology departments segment is expected to witness a significant rate of expansion during the forecast period.
The Atherectomy Devices Market is witnessing robust growth driven by the increasing prevalence of cardiovascular diseases and the growing demand for minimally invasive procedures. Atherectomy devices play a pivotal role in the removal of atherosclerotic plaques from blood vessels, thereby restoring blood flow and preventing complications such as heart attacks and strokes. As the aging population continues to expand globally, there is a rising incidence of atherosclerosis-related conditions, fueling the demand for advanced atherectomy devices.
Technological advancements in atherectomy devices have paved the way for more efficient and safer procedures. The development of innovative catheter-based atherectomy systems, guided by imaging technologies such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), has enhanced precision and reduced procedural risks. Moreover, the shift towards outpatient settings and the increasing adoption of minimally invasive interventions contribute significantly to the market’s growth.
Get a Sample: https://www.precedenceresearch.com/sample/3901
Growth Factors
- Prevalence of Cardiovascular Diseases: The escalating global burden of cardiovascular diseases, including coronary artery disease, peripheral artery disease, and other vascular disorders, is a primary driver for the growing demand for atherectomy devices. These conditions necessitate effective and less invasive treatments, boosting the adoption of atherectomy procedures.
- Technological Advancements: Ongoing technological innovations in atherectomy devices, such as the incorporation of laser and rotational atherectomy techniques, enhance their efficacy and safety. These advancements attract both healthcare providers and patients, fostering the market’s expansion.
- Aging Population: The increasing geriatric population, particularly in developed regions, contributes significantly to the prevalence of atherosclerosis-related conditions. As elderly individuals are more prone to vascular diseases, the demand for atherectomy devices is expected to rise correspondingly.
- Outpatient Treatment Trends: The shift towards outpatient settings for cardiovascular interventions is gaining momentum. Atherectomy procedures performed in outpatient facilities offer advantages such as reduced hospitalization costs and quicker recovery times, further propelling market growth.
Regional Analysis
The Atherectomy Devices Market exhibits regional variations influenced by factors such as healthcare infrastructure, economic development, and disease prevalence.
- North America: With a well-established healthcare system and a high prevalence of cardiovascular diseases, North America dominates the atherectomy devices market. The region benefits from early technology adoption and significant investments in research and development.
- Europe: European countries experience a substantial burden of cardiovascular diseases, contributing to the growth of the atherectomy devices market. The presence of key market players and favorable reimbursement policies further drive market expansion in this region.
- Asia-Pacific: The Asia-Pacific region is witnessing rapid market growth due to the increasing awareness of cardiovascular health, rising disposable incomes, and improvements in healthcare infrastructure. Emerging economies in this region present lucrative opportunities for atherectomy device manufacturers.
- Latin America and Middle East/Africa: These regions are experiencing a gradual increase in the prevalence of cardiovascular diseases, creating a growing market for atherectomy devices. Market players are focusing on expanding their presence and tapping into the untapped potential in these regions.
Read Also: Green Cement Market Size to Rise USD 1,046.76 Million by 2033
SWOT Analysis:
Strengths:
- Technological Innovation: Atherectomy device manufacturers are continually investing in research and development, leading to the introduction of technologically advanced and innovative products.
- Increasing Patient Preference for Minimally Invasive Procedures: The trend towards minimally invasive interventions benefits atherectomy devices, as patients seek procedures with shorter recovery times and reduced complications.
Weaknesses:
- High Initial Costs: The initial costs associated with acquiring atherectomy devices can be relatively high, posing a challenge, especially for healthcare facilities with budget constraints.
- Limited Awareness: In certain regions, there is a lack of awareness among both healthcare providers and patients about the benefits of atherectomy procedures, hindering market growth.
Opportunities:
- Emerging Markets: Untapped markets in developing regions present significant growth opportunities for atherectomy device manufacturers, as these areas witness an increasing incidence of cardiovascular diseases.
- Collaborations and Partnerships: Forming strategic alliances with healthcare providers and institutions can enhance market penetration and facilitate the development of tailored solutions.
Threats:
- Stringent Regulatory Approval Processes: Stringent regulatory processes can pose challenges for new entrants and hinder the timely launch of innovative atherectomy devices.
- Competition from Alternative Therapies: Atherectomy devices face competition from alternative treatment modalities, such as drug therapies and traditional open surgeries, which may limit market growth.
Recent Developments
- In October 2023, Cardio Flow, Inc., announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its FreedomFlow® Orbital Atherectomy Peripheral Platform.
- In September 2023, Avinger Inc., a commercial-stage medical device company, announced the full commercial launch of its Tigereye® ST next-generation image-guided chronic total occlusion (CTO) crossing system.
Atherectomy Devices Market Companies
- Abbott Laboratories
- Boston Scientific Corporation
- BD
- Cardinal Health Inc.
- Koninklijke Philips NV
- Medtronic Plc
- Terumo Corporation
- Avinger
- Cardiovascular Systems
- Ra Medical Systems
Segments Covered in the Report
By Devices Type
- Atherectomy Devices
- Angioplasty Balloon Catheters
- Stents (Bare-Metal, Drug-Eluting, Bioresorbable)
- Intravascular Ultrasound (IVUS) Catheters
- Optical Coherence Tomography (OCT) Catheters
- Drug-Coated Balloons
- Embolic Protection Devices
- Thrombectomy Devices
- Aortic Stent Grafts
- Endovascular Grafts
- Laser Atherectomy Devices
- Orbital Atherectomy Systems
- Rotational Atherectomy Devices
- Directional Atherectomy Devices
- Chronic Total Occlusion (CTO) Devices
By Application
- Peripheral Artery Disease (PAD) Treatment Devices
- Coronary Artery Disease (CAD) Intervention Devices
- Carotid Artery Disease Intervention Devices
- Renal Artery Disease Intervention Devices
- Aortic Atherosclerosis Intervention Devices
By End-user
- Cardiac Catheterization Labs
- Interventional Radiology Departments
- Vascular Surgery Centers
- Cardiology Clinics
- Academic Research Institutions
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
