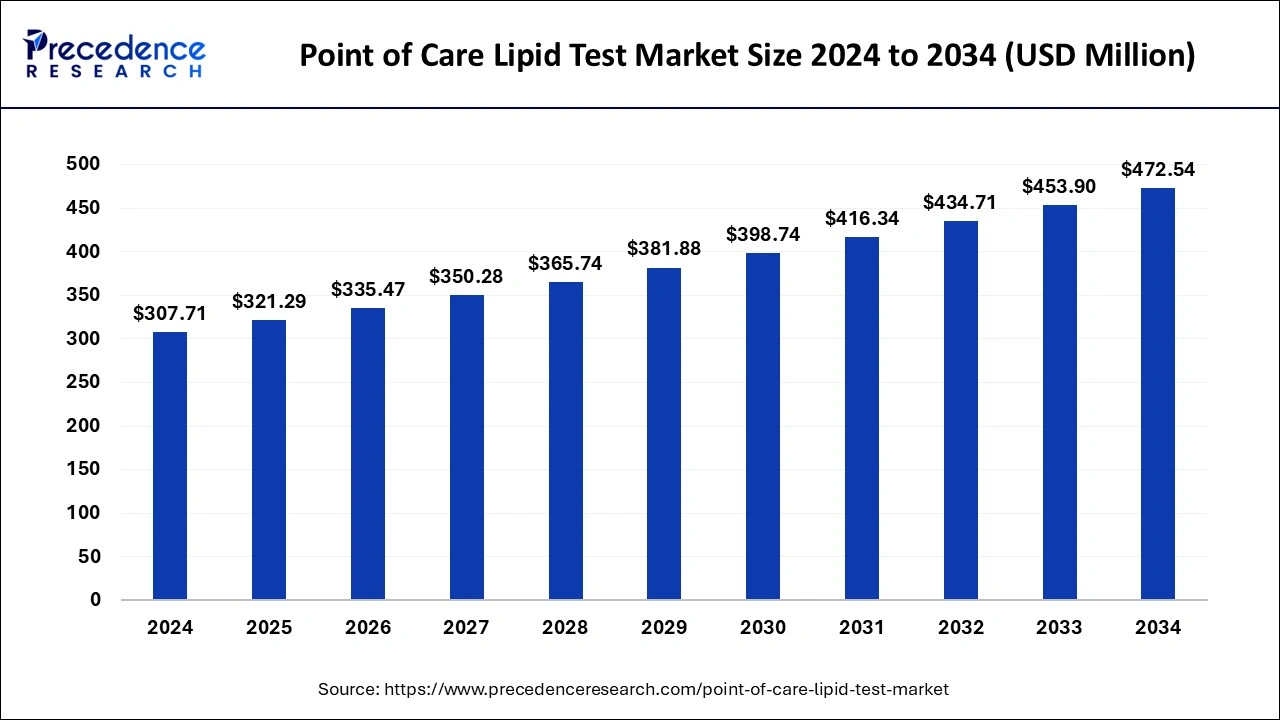
The global point of care lipid test market size is expected to increase USD 453.90 million by 2033 from USD 294.70 million in 2023 with a CAGR of 4.41% between 2024 and 2033.
Key Points
- North America held a significant share of in the point of care lipid test market in 2023 with 38.6%.
- Asia Pacific is anticipated to experience the highest growth in the upcoming years.
- By product, the consumables type segment has accounted market share of 57% in 2023.
- By application, in 2023, the endogenous hyperlipidemia segment dominated the market with 46% revenue share.
- By end use, the diagnostic centers segment has held largest market share of 56% in 2023.
The Point of Care (POC) Lipid Test Market encompasses diagnostic tests designed for immediate or near-patient use, providing quick and reliable results to measure lipid levels, including cholesterol, triglycerides, and lipoproteins. These tests play a crucial role in monitoring cardiovascular health and managing diseases such as hyperlipidemia and atherosclerosis. POC lipid tests offer the advantage of convenience and rapid results, allowing healthcare providers to make real-time decisions regarding patient care. The market has experienced significant growth in recent years due to rising awareness of cardiovascular diseases and the increasing prevalence of conditions such as obesity and diabetes.
Get a Sample: https://www.precedenceresearch.com/sample/4076
Growth Factors
- Rising Prevalence of Cardiovascular Diseases: The increasing incidence of heart-related diseases globally has driven the demand for POC lipid tests. Early diagnosis and regular monitoring are essential in managing these conditions.
- Advancements in POC Testing Technology: Technological advancements have led to more precise and efficient testing methods. Portable devices with user-friendly interfaces and connectivity options for data sharing are becoming more common.
- Increasing Geriatric Population: The aging population is more prone to cardiovascular diseases, which in turn drives the demand for POC lipid tests for routine health check-ups and monitoring.
- Awareness and Preventive Healthcare: Growing awareness of the importance of monitoring lipid levels for overall health and preventing heart diseases has increased the demand for POC lipid testing.
- Cost-Effective Testing Solutions: POC tests offer a more affordable alternative to traditional lab-based testing, reducing the overall cost of healthcare.
Region Insights
- North America: The region leads the market due to advanced healthcare infrastructure, high awareness of preventive healthcare, and government initiatives promoting regular health check-ups.
- Europe: Europe follows North America in terms of market share, supported by a well-established healthcare system and increasing awareness of the benefits of early diagnosis and monitoring.
- Asia-Pacific: This region is witnessing rapid market growth due to the increasing prevalence of cardiovascular diseases, a large population, and improving healthcare infrastructure.
- Latin America and Middle East & Africa: These regions are expected to experience moderate growth as healthcare infrastructure improves and awareness of cardiovascular diseases increases.
Point of Care Lipid Test Market Scope
| Report Coverage | Details |
| Global Market Size in 2023 | USD 294.70 Million |
| Global Market Size in 2024 | USD 307.71 Million |
| Global Market Size by 2033 | USD 453.90 Million |
| Growth Rate from 2024 to 2033 | CAGR of 4.41% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product Type, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Point of Care Lipid Test Market Dynamics
Drivers
- Technological Innovation: Advancements in technology are making POC lipid tests more accurate, user-friendly, and efficient, which is driving market growth.
- Government Initiatives and Policies: Government programs promoting preventive healthcare and regular screening for cardiovascular diseases are boosting the demand for POC lipid tests.
- Increasing Adoption of Home Healthcare: As more people adopt home healthcare options, the demand for POC lipid tests for home use is increasing.
Opportunities
- Expansion in Emerging Markets: Emerging markets, particularly in Asia and Africa, present significant growth opportunities as healthcare infrastructure improves and awareness of cardiovascular health increases.
- Partnerships and Collaborations: Collaborations between healthcare providers and diagnostic companies can lead to the development of more advanced POC lipid tests and broaden market reach.
- Integration with Telehealth: Integrating POC lipid tests with telehealth services can enhance patient care and expand the market by providing remote monitoring and consultation options.
Challenges
- Regulatory Hurdles: Stringent regulatory requirements and approval processes for medical devices can delay the introduction of new POC lipid tests to the market.
- Cost and Affordability: While POC lipid tests are cost-effective, affordability may still be a concern in certain regions, potentially limiting market growth.
- Competition from Laboratory Testing: Traditional laboratory testing remains a competitor due to its established presence and perceived reliability, which may slow the adoption of POC lipid tests.
Read Also: Electric Vehicle Thermal Management Market Size, Trends, Report By 2033
Recent Developments
- In October 2022 Genes2Me Pvt. Ltd launched Rapi-Q- Point of Care RT PCR solution for human papillomavirus (HPV) and tuberculosis. The device is easy to use and gives faster results in less than 45 minutes. This CE-IVD-marked POC solution delivers superior performance, high sensitivity, and stable detection.
- In March 2022, Visby Medical announced that it received funding of USD 25.5 million from the U.S. Biomedical Advanced Research and Development Authority to develop a rapid flu-COVID-19 PCR test for home use. At present, the test is in the under-developing phase, and the design is ready as a PCR device that can detect COVID-19, influenza A, and B from a single sample.
- In March 2022, Canada-based Company BioLytical Laboratories Inc. received a CE marking for the iStatis COVID-19 Antigen Home Test.
- In May 2022, Qiagen Inc. launched the NeuMoDxHSV 1/2 Quant Assay for the quantification and differentiation of herpes simplex virus type 1 (HSV-1) DNA and herpes simplex virus type 2 (HSV-2) with approval from the European Commission. The emergence of this assay is allowing the company to expand its product portfolio in laboratory testing, which ultimately helps the market grow owing to the innovative tests.
- In March 2022, Mindray launched the BC-700 Series, a hematology analyzer series that assists in both blood count and erythrocyte sedimentation rate tests.
Point of Care Lipid Test Market Com[panies
- Callegari Sinocare Inc.
- Abbott Laboratories
- Mico Bio Med
- Nova Biomedical Corporation
- VivaChek Biotech (Hangzhou) Co., Ltd.
- F. Hoffmann-La Roche Ltd.
- Zoetis Inc.
- Menarini Group
- SD Biosensor, Inc.
Segments Covered in the Report
By Product Type
- Devices
- Consumables
By Application
- Endogenous Hyperlipemia
- Combined Hyperlipidemia
- Familial Hypercholesterolemia
- Others
By End-user
- Hospitals And Clinics
- Diagnostic Laboratories
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
