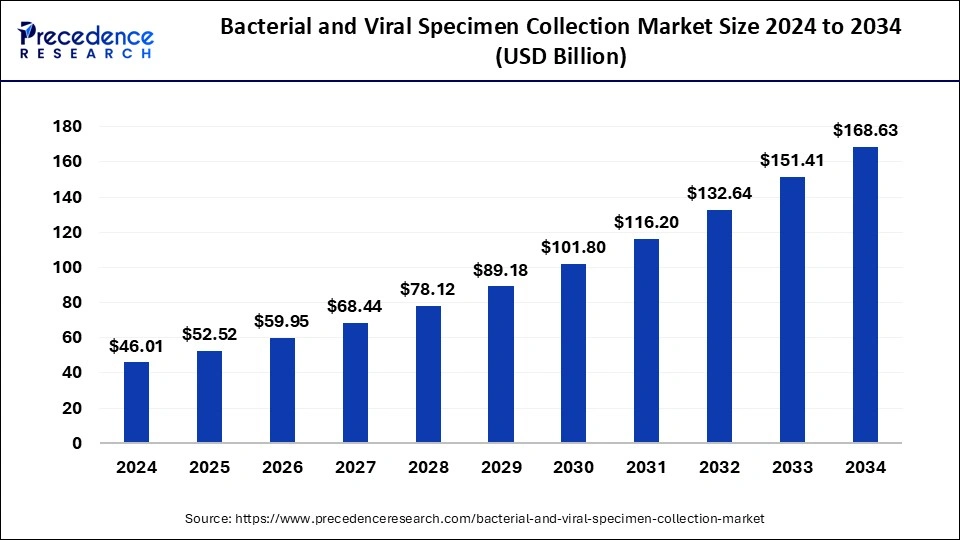
The global bacterial and viral specimen collection market size reached USD 40.31 billion in 2023 and is expected to surpass around USD 151.41 billion by 2033. at a solid CAGR of 14.15% from 2024 to 2033.
Key Points
- The North America bacterial and viral specimen collection market size reached USD 18.54 billion in 2023 and is expected to attain around USD 70.41 billion by 2033, poised to grow at a CAGR of 14.27% between 2024 and 2033.
- North America dominated the global market with the largest revenue share of 46% in 2023.
- Asia Pacific is expected to grow at a significant rate in the market during the forecast period.
- By product, the bacterial specimen collection segment has held the biggest revenue share of 62% in 2023.
- By application, the diagnostic has contributed more than 74% of revenue share in 2023.
- By end user, the hospitals and clinics segment has recorded more than 61% of revenue share in 2023.
The Bacterial and Viral Specimen Collection Market is a crucial segment within the broader diagnostic and healthcare landscape, focusing on the tools and products used to collect, transport, and preserve specimens for diagnosing bacterial and viral infections. These products include swabs, collection tubes, transport media, and specialized containers designed to maintain the integrity and viability of samples from the point of collection to the laboratory. The demand for effective specimen collection solutions has surged in recent years, driven by the increasing prevalence of infectious diseases, the need for accurate diagnostic results, and advancements in medical research and technology. This market plays a vital role in ensuring that healthcare providers can accurately diagnose and treat infectious diseases, ultimately improving patient outcomes and public health.
Get a Sample: https://www.precedenceresearch.com/sample/4594
Growth Factors
Several key factors are driving the growth of the bacterial and viral specimen collection market. One of the primary drivers is the increasing incidence of infectious diseases worldwide. Outbreaks of diseases such as COVID-19, Ebola, and influenza have highlighted the need for reliable specimen collection methods to quickly identify and contain these threats. Additionally, advancements in diagnostic technologies, including molecular diagnostics and rapid testing, have increased the demand for high-quality specimen collection products that can preserve the integrity of samples for accurate analysis.
Another significant growth factor is the growing awareness and implementation of regular screening programs for infectious diseases. Public health initiatives aimed at early detection and prevention of diseases are encouraging more frequent and widespread use of specimen collection devices. Furthermore, rising healthcare expenditure globally and improvements in healthcare infrastructure, particularly in emerging markets, are supporting the expansion of diagnostic capabilities and the adoption of advanced specimen collection solutions.
Regional Insights
The market for bacterial and viral specimen collection exhibits significant regional variations, influenced by factors such as healthcare infrastructure, disease prevalence, and economic development. North America dominates the market, driven by a well-established healthcare system, high adoption of advanced diagnostic technologies, and proactive government initiatives focused on disease surveillance and control. The region’s robust research and development activities and strong regulatory frameworks further bolster market growth.
Europe also holds a substantial market share, supported by increasing healthcare expenditure, stringent regulatory standards, and a strong emphasis on research and innovation. The presence of major diagnostic companies and extensive healthcare networks contribute to the region’s market strength.
The Asia-Pacific region is emerging as a lucrative market for bacterial and viral specimen collection. Rapid urbanization, increasing healthcare awareness, and expanding healthcare access in countries like China and India are driving demand. Investments in healthcare infrastructure and diagnostic laboratories are also propelling market growth in this region. Latin America and the Middle East/Africa are witnessing moderate growth, with improving healthcare facilities, rising disposable incomes, and government initiatives aimed at controlling infectious diseases contributing to market expansion.
Bacterial and Viral Specimen Collection Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 151.41 Billion |
| Market Size in 2023 | USD 40.31 Billion |
| Market Size in 2024 | USD 46.01 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 14.15% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Product, Application, End-use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Bacterial and Viral Specimen Collection Market Dynamics
Drivers
Several key drivers are propelling the growth of the bacterial and viral specimen collection market. Technological advancements in specimen collection devices are playing a crucial role. Innovations aimed at improving sample preservation and transport capabilities are enhancing the reliability and accuracy of diagnostic tests. These advancements include the development of new types of swabs, collection containers with advanced preservatives, and integrated systems that streamline the collection and transport process.
The ongoing focus on pandemic preparedness is another significant driver. Global outbreaks, such as the COVID-19 pandemic, have underscored the importance of robust specimen collection systems. This has led to increased investments in diagnostic capabilities and the development of standardized, high-quality collection devices to ensure the effective management of future outbreaks.
Increasing diagnostic testing for infectious diseases, driven by growing population awareness and improvements in healthcare infrastructure, is also fueling market growth. The expansion of diagnostic laboratories and the implementation of widespread screening programs are boosting demand for specimen collection devices. Additionally, regulatory support in the form of stringent guidelines for the use of standardized collection devices is ensuring diagnostic accuracy and patient safety, further driving market growth.
Opportunities
The bacterial and viral specimen collection market presents several opportunities for growth and innovation. One significant opportunity lies in the expansion into emerging markets. Developing regions with improving healthcare infrastructure and rising healthcare awareness offer untapped potential for market players. Establishing distribution networks and increasing market presence in these regions can drive substantial growth.
Product diversification is another key opportunity. The development and launch of advanced specimen collection devices tailored to specific diagnostic needs, such as rapid testing kits and integrated sample collection systems, can cater to a broader range of healthcare providers and diagnostic applications. Strategic collaborations and partnerships between healthcare providers, diagnostic laboratories, and manufacturers also present opportunities to enhance product development, distribution capabilities, and market reach.
The increasing adoption of telemedicine and remote testing creates opportunities for specimen collection devices designed for home healthcare settings. As telemedicine platforms gain popularity, the need for reliable and user-friendly collection devices that can be used by patients at home is growing. This trend opens new avenues for innovation and market expansion.
Challenges
Despite the promising growth prospects, the bacterial and viral specimen collection market faces several challenges. One of the primary challenges is the high cost associated with the development and commercialization of advanced specimen collection devices. Significant research and development investments are required to create innovative products that meet regulatory standards and market demands, impacting pricing and market accessibility.
Logistical challenges also pose a significant hurdle. Ensuring reliable transportation and storage conditions to maintain sample integrity during transit, particularly in remote and resource-limited settings, is crucial. Variations in infrastructure and logistical capabilities across different regions can affect the timely and efficient delivery of collected specimens to testing laboratories.
Regulatory hurdles present another challenge for market players. Compliance with diverse regulatory requirements across different regions can delay product approvals and complicate market entry strategies. Navigating the complex regulatory landscape requires significant resources and expertise, impacting the speed and efficiency of bringing new products to market.
Global supply chain disruptions, such as those experienced during the COVID-19 pandemic, can also affect the availability and distribution of specimen collection devices. Interruptions in the supply of raw materials, manufacturing processes, and distribution networks can lead to shortages and delays, impacting market growth and stability.
Read Also: Advanced Li-ion Battery Technologies Market Size, Growth, Report 2033
Bacterial and Viral Specimen Collection Market Key Companies
- Puritan Medical Products
- COPAN Diagnostics
- Becton, Dickinson and Company
- Thermo Fisher Scientific, Inc.
- Quidel Corporation
- Longhorn Vaccines and Diagnostics, LLC
- Pretium Packaging
- Trinity Biotech
- Medical Wire & Equipment
- HiMedia Laboratories
- Hardy Diagnostics
- Nest Scientific
- VIRCELL S.L.
- DiaSorin
- Titan Biotech
Recent Developments
- On June 04, 2024, according to a study published in The Lancet, a Peer-reviewed journal and the European Health Union, European government faculty revealed that the 29-year-old Dutch scientist Rochelle Niemeijer innovated an affordable, fast, and artificial intelligence-driven tool to collect and diagnose bacterial infectious specimens. Up until July 9, 2024, the Dutch innovator will compete for the Young Inventors Prize, a recognition established by the European Patent Office, against a team from Tunisia and a finalist from Ukraine. Due to her promising work addressing one of the Sustainable Development Goals (SDGs) of the United Nations by making specimen collection and diagnosis more accessible, Niemeijer has been nominated as a finalist for the Young Inventors Prize of the European Inventor Award 2024.
- On January 19, 2024, ELITechGroup, an in-vitro diagnostics company, revealed the launch of the ‘GI Bacterial PLUS ELITe MGB Kit,’ which assists in bacterial specimen collection and diagnosis. The in vitro assay made available in ‘GI Bacterial PLUS ELITe MGB Kit’ is designed specifically to identify bacterial infections in the gastrointestinal tract. It targets key pathogens such as Yersinia enterocolitica, Clostridium difficile, Shigella species, Campylobacter species, and Salmonella species.
Segments Covered in the Report
By Product
- Bacterial Specimen Collection
- Swabs
- Bacterial Transport Media
- Blood Collection Kits
- Other Consumables
- Viral Specimen Collection
- Swabs
- Viral Transport Media
- Blood Collection Kits
- Other Consumables
By Application
- Diagnostics
- Research
By End-use
- Hospitals & Clinics
- Home Test
- Research Laboratories
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
