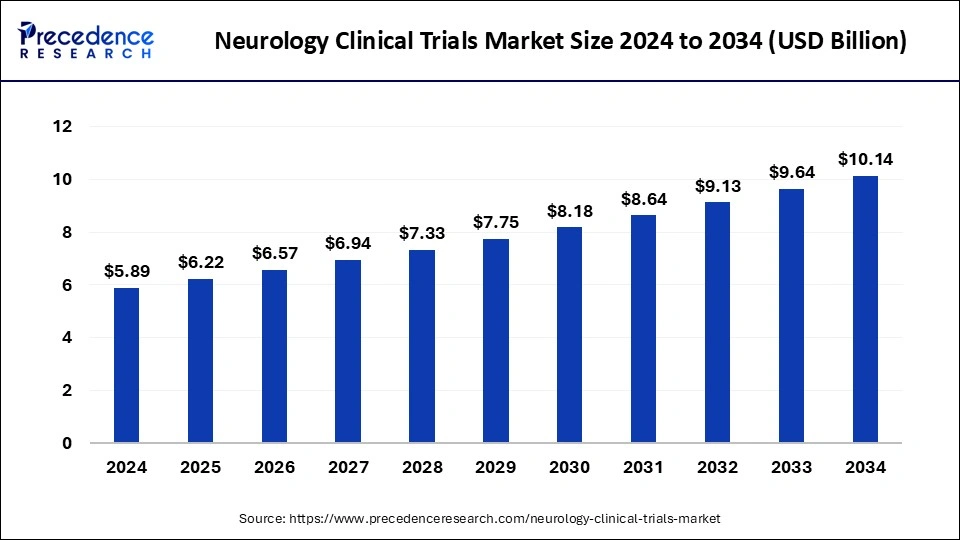
The global neurology clinical trials market size is estimated at USD 5.89 billion in 2024 and is projected to cross around USD 9.64 billion by 2033 with a notable CAGR of 5.62% from 2024 to 2033.
Neurology Clinical Trials Market Key Statistics
- North America dominated the neurology clinical trials market with the largest revenue share of 48% in 2023.
- Asia Pacific is estimated to grow at a solid CAGR of 5.93% during the forecast period of 2024-2033.
- By phase, the phase II segment has generated more than 47% of revenue share in 2023.
- By phase, the phase III segment is projected to expand at a CAGR of 5.62% during the forecast period.
- By indication, the epilepsy segment has recorded more than 23% of revenue share in 2023.
- By indication, the huntington’s disease segment growing at a CAGR of 6.04% during the forecast period.
- By study design, the interventional segment dominated the market with the biggest revenue share of 96% in 2023.
- By study design, the observational segment is expected to grow at a CAGR of 5.83% during the forecast period.
The Neurology Clinical Trials market encompasses a broad spectrum of trials focused on neurological disorders, ranging from Alzheimer’s disease and Parkinson’s disease to epilepsy and multiple sclerosis. These trials play a crucial role in advancing medical understanding and treatment options for patients suffering from these complex conditions. The market for neurology clinical trials is driven by increasing incidences of neurological disorders globally, coupled with growing investments in research and development by pharmaceutical companies and academic institutions.
Get a Sample:https://www.precedenceresearch.com/sample/4657
Region Insights
North America dominates the neurology clinical trials market, driven by robust healthcare infrastructure, high R&D expenditure, and a significant presence of pharmaceutical giants focusing on neurological disorders. Europe follows closely, buoyed by supportive regulatory frameworks and increasing government initiatives to promote clinical research. Meanwhile, Asia-Pacific is emerging as a lucrative region, characterized by rising healthcare expenditure, expanding patient population, and improving clinical trial capabilities.
Trends
- Increasing Neurological Disorders: With a rise in neurological disorders such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, there is a growing need for clinical trials to develop new treatments and therapies.
- Advancements in Biomarkers and Imaging Technologies: Biomarkers and advanced imaging techniques like MRI and PET scans are increasingly being used to improve diagnosis, patient selection, and monitoring of neurological conditions in clinical trials.
- Focus on Precision Medicine: There is a shift towards personalized or precision medicine in neurology, where treatments are tailored to individual genetic, molecular, and environmental factors. This trend is driving the need for clinical trials that target specific patient subgroups.
- Rise of Virtual and Decentralized Trials: Virtual and decentralized clinical trials are becoming more prevalent in neurology research, allowing for remote patient monitoring and data collection. This approach enhances patient participation and diversity in trials while reducing costs and logistical challenges.
- Innovative Therapeutic Approaches: The field is witnessing innovation in therapeutic approaches, including gene therapy, stem cell therapy, and novel drug delivery systems. Clinical trials are crucial for evaluating the safety and efficacy of these cutting-edge treatments.
Neurology Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 9.64 Billion |
| Market Size in 2023 | USD 5.58 Billion |
| Market Size in 2024 | USD 5.89 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 5.62% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Indication, Study Design, Phase, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Phase Insights
Neurology clinical trials progress through several distinct phases, each serving specific objectives crucial for drug development and regulatory approval. Phase 1 trials typically involve a small group of healthy volunteers or patients and focus on assessing the safety, dosage, and pharmacokinetics of new treatments. These initial studies are essential for understanding how the drug behaves in the body and identifying potential side effects. Phase 2 trials expand to a larger group of patients diagnosed with specific neurological conditions such as Alzheimer’s disease, multiple sclerosis, or Parkinson’s disease. Here, researchers evaluate the preliminary efficacy and further assess safety profiles. Phase 3 trials are pivotal, involving a larger patient population to rigorously evaluate the treatment’s effectiveness compared to existing therapies or placebos. These trials generate robust data essential for regulatory submissions and approval. Finally, Phase 4 trials occur post-marketing and focus on long-term safety, optimal usage, and real-world effectiveness in broader patient populations, providing crucial insights into the treatment’s performance over time.
Study Design Insights
Study design in neurology clinical trials varies based on the research objectives and the nature of the treatment being evaluated. Randomized Controlled Trials (RCTs) are commonly employed to minimize bias by randomly assigning participants to treatment groups. This method is crucial for assessing the efficacy of new drugs against placebos or standard treatments. Observational studies provide valuable insights into treatment outcomes in real-world settings, particularly for chronic conditions like epilepsy or stroke where long-term effects are significant. Crossover trials allow participants to receive different treatments sequentially, serving as their own controls, which can be useful in conditions with fluctuating symptoms like migraine. Open-label trials involve both participants and researchers knowing which treatment is administered, facilitating ethical considerations or when blinding isn’t feasible, such as in surgical trials or certain rehabilitation studies.
Indications Insights
Neurology clinical trials encompass a broad spectrum of indications addressing various neurological disorders. Alzheimer’s disease and dementia trials focus on disease-modifying therapies aimed at slowing cognitive decline and improving quality of life for patients and caregivers. Multiple sclerosis trials seek to develop new disease-modifying drugs that can delay progression and manage symptoms like fatigue and motor impairment. Parkinson’s disease research includes trials for therapies targeting motor symptoms, tremors, and non-motor symptoms such as depression and sleep disturbances. Stroke clinical trials investigate acute treatments to minimize brain damage and long-term rehabilitation strategies to improve functional outcomes. Epilepsy trials explore new antiepileptic drugs, surgical interventions, and devices to control seizures effectively and improve patient quality of life.
Neurology Clinical Trials Market Dynamics
Drivers
The primary drivers of the neurology clinical trials market include increasing prevalence of neurological disorders worldwide, prompting heightened research activities. Moreover, advancements in neuroscience and biotechnology are accelerating drug discovery and development processes. Additionally, regulatory reforms aimed at expediting trial timelines and enhancing patient recruitment are facilitating market growth. Furthermore, growing investments from both public and private sectors are fueling innovation and expanding the scope of clinical trials in neurology.
Opportunities
Opportunities abound in the neurology clinical trials market, driven by expanding research into rare neurological diseases and novel therapeutic approaches. Collaborations between academia, pharmaceutical companies, and research organizations are fostering innovation and accelerating clinical trial pipelines. Moreover, the advent of artificial intelligence and machine learning is revolutionizing data analytics, offering new avenues for predictive modeling and patient stratification in neurology trials.
Challenges
Despite its promise, the neurology clinical trials market faces several challenges. These include high costs associated with drug development, stringent regulatory requirements, and ethical considerations in patient recruitment and data privacy. Furthermore, the complexity of neurological disorders poses challenges in trial design and endpoint selection, necessitating robust methodologies and interdisciplinary collaboration. Moreover, disparities in healthcare access and patient diversity across regions can impact the generalizability of trial results and limit global market penetration.
Read Also: Menstrual Health Apps Market Size to Attain USD 9.04 Bn by 2033
Recent Developments
- In August 2023, the phase II RECOVER-NEURO clinical trial study to evaluate the combination of REMOTE-transcranial direct current stimulation (tDCS) and a brain training program for long covid was launched by Soterix Medical.
- In March 2024, an innovative and new pTau217 blood test, ALZpath Dx, was launched by a specialized clinical laboratory, Neurocode USA, Inc., that offers world-class testing solutions for neurological disorders. This new test may be used in the monitoring, screening, and diagnosis of Alzheimer’s disease. In the US, Neurocode is the first laboratory to make this test as LDT (laboratory-developed test) for clinical trials, clinical diagnostics use, and other research causes.
Neurology Clinical Trials Market Companies
- IQVIA
- Biogen
- Aurora Healthcare
- GlaxoSmithKline Plc.
- Icon Plc.
- Syneous Health
- Charles River Laboratories
- Med pace
- Covance
- Novartis AG
- Sanofi
- Merck & Co., Inc.
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Annovis Bio
- Athira Pharma, Inc.
- Zydus Group
- Eli Lilly and Company
- Eisai Co., Ltd.
- AstraZeneca
- Supernus Pharmaceuticals, Inc. (Adamas Pharmaceuticals)
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
