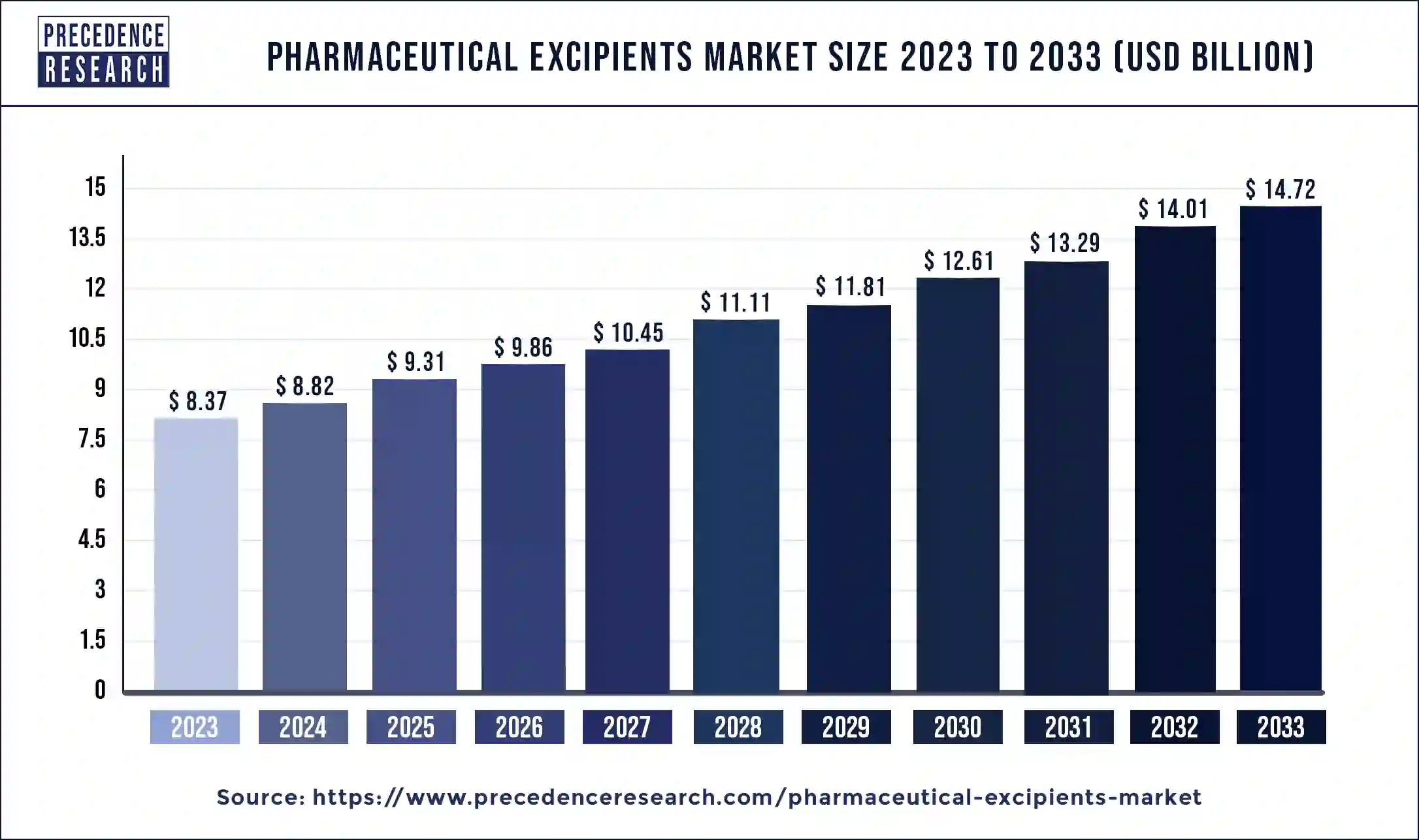
The global pharmaceutical excipients market size reached USD 8.37 billion in 2023 and is predicted to grow around USD 14.72 billion by 2033, at a CAGR of 5.81% from 2024 to 2033.
Pharmaceutical Excipients Market Key Insights:
- North America dominated the pharmaceutical excipients market with the largest market share of 38.32% in 2023.
- By excipient type, the lactose-based excipients has held the highest market share of 41.26% in 2023.
- By functionality, the binders & fillers has generated the largest market share of 49.72% in 2023.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/1761
The pharmaceutical excipients market comprises the global industry involved in the production and supply of inactive ingredients used in pharmaceutical formulations. Pharmaceutical excipients are compounds that are not active pharmaceutical ingredients (APIs) but are deliberately included in a medication delivery system. They allow the drug ingredient to be administered to the patient in the appropriate form and support the method and location of action without being active themselves. Excipients can be inert and simple or active and complex.
Historically, excipients were biologically inert, structurally simple, and naturally derived. However, several excipients are now considered potential toxins at large levels in animals but safe in humans at therapeutic amounts. Pharmaceutical-grade excipients must meet pharmacopeias such as USP-NF, Ph. Eur, and JP, as well as GMP production standards. Excipients have a variety of roles in a formulation, including aiding in protecting stability, drug delivery system processing, improving product identification, increasing therapeutic effectiveness, and preserving drug product integrity during storage.
The projections for growth by region show:
- The North America pharmaceutical excipients market size was valued at USD 3.15 billion in 2023 and is projected to surpass around USD 5.66 billion by 2033, growing at a CAGR of 6.04% from 2024 to 2033.
- The Europe pharmaceutical excipients market size reached USD 2.35 billion in 2023 and is predicted to hit around USD 3.95 billion by 2033, growing at a CAGR of 5.30% from 2024 to 2033.
- The Asia Pacific pharmaceutical excipients market size was estimated at USD 1.94 billion in 2023 and is expected to grow at a CAGR of 6.53% from 2024 to 2033, to reach around USD 3.65 billion by 2033.
- The MEA pharmaceutical excipients market size is expected to be worth around USD 454.85 million by 2033 from USD 294.40 million in 2023 and expanding at a CAGR of 4.44% from 2024 to 2033.
Regional Snapshot
North America dominated the pharmaceutical excipients market in 2023. The North American pharmaceutical excipients industry is developing due to increased generics and product launches. Governments in poor nations are increasing the manufacture of generic medicine to lower healthcare costs and protect branded drug patents. This growth has resulted in above-average shareholder returns. Excipients influence bioavailability, dosage structure, stability, patient acceptability, and efficacy. The region’s rising generic medicine sales increase demand for these excipients.
For instance, CPHI North America, which will take place from May 7-9, 2024, serves as a hub for the world’s largest healthcare economy, pharmaceutical markets, ingredient suppliers, and the full supply chain. The event brings together industry leaders, innovators, and developers to demonstrate cutting-edge advances in the pharmaceutical business. The Exploring Excipients track emphasizes the crucial role of excipients in medication development, with keynote speakers addressing the significance of excipient grades.
Asia-Pacific is expected to experience the fastest growth in 2023. The market for pharmaceutical excipients is predicted to expand due to rising demand for alternative delivery methods and pharmaceutical products in various therapeutic areas. Advanced drug delivery technology, such as controlled-release formulations and transdermal patches, necessitate the use of specialized excipients to achieve desired release patterns and therapeutic effects. This need is fueled by the ongoing discovery of new medications and the need for novel drug delivery technologies.
Rising research and development costs for pharmaceutical products and diagnostic procedures, as well as higher expenditures in healthcare facilities, drive market expansion. Furthermore, industry partnerships and collaborations for tailored excipients are expected to aid market growth in the coming years, according to the report. The market is predicted to expand due to rising demand for drugs across a variety of therapeutic areas.
Pharmaceutical Excipients Market Segment Outlook:
Functionality Outlook
Fillers & diluents dominated the pharmaceutical excipients market in 2023. Pharmaceutical formulations include fillers for a variety of reasons, including filler shape, concentration, physiological inertness, bulk density, particle size, aspect ratio, low API binding capacity, processability, strength, and cost-effectiveness, physical and chemical stability. Compressibility, compatibility, flowability, particle size, cost and availability, moisture content, abrasiveness, stability, solubility, bulk density, physiological inertness, and regulatory acceptance all play important roles in solid dosage forms.
Pharmaceutical fillers give structure, influence appearance, and have an impact on other measurable aspects that indicate a product’s intended use. Lactose and microcrystalline cellulose are the most often used diluents for solid dosage forms. Mannitol is used as a replacement, although it is expensive and only justified when other functional qualities are desired in a formulation.
Excipient Type Outlook
The lactose-based excipients segment dominated the pharmaceutical excipients market with the largest market share in 2023. Lactose monohydrate is a popular excipient in the pharmaceutical business, serving as a filler and diluent in solid dosage forms, a lyophilization aid in parenteral products, a filler and carrier in inhalation products, and a nutrient and filler in infant formula. Its features include a highly defined material, acceptable physicochemical qualities, odorless taste, widespread availability, low hygroscopicity and stability, low cost, and compatibility with a wide range of active medicinal ingredients and excipients.
A cellulose-based segment is expected to grow at the fastest rate during the forecast period. Microcrystalline cellulose is a popular excipient in the pharmaceutical industry due to its superior compressibility and application in solid dosage forms such as tablets. It fulfills USP criteria and is used in processed foods as a stabilizer, an anti-caking agent, a suspending agent, or a texture adjuster. The Select Committee on GRAS Substances typically finds microcrystalline cellulose to be safe when used in regular quantities.
Read Also: Wind Energy Market Size to Hit USD 260.81 Bn by 2034
Pharmaceutical Excipients Market Dynamics
Driver: The good stabilizing power of excipients
Stabilization techniques for therapeutic products include spectral overlay, antioxidants in formulations prone to oxidative breakdown, and excipients that prevent group interaction in nearby molecules or vehicles. Chelating agents, preservatives, polyethylene glycol, buffers, and cyclodextrins are among the tactics used. The spectral overlay strategy entails creating a formulation with an excipient whose light absorption spectrum overlaps that of the photolabile medication.
Vitamin-containing goods include antioxidants, whereas excipients prevent group interaction in nearby molecules or vehicles. Preservatives inhibit microbiological development in liquid products, buffers create a pH-dependent environment that promotes stability, and chelating agents prohibit heavy metals from accelerating deterioration.
Restraint: Strict safety assessment
The safety of excipients in medicinal products has been a sensitive issue in public discourse, with some misconceptions spreading. Regulatory bodies require producers to conduct extensive testing before utilizing any item as an excipient. Excipients were once thought to be innocuous and infrequently controlled, resulting in severe public harm.
However, as products and components become more complicated, excipient safety testing has become required. The US FDA, MHRA, and EMA have approved excipient lists, which include pharmacopeia monographs, databases, and manufacturing and distribution guidelines. Manufacturers must follow these guidelines to ensure the safety of their products.
Opportunity: Excipient-loaded platform technology
Creating an excipient-loaded platform technology for pharmaceutical final dosage forms is a great opportunity for the pharmaceutical excipients market. The technology intends to pack active compounds into an orally dispersible tablet (ODT) that dissolves quickly and clings to the mucosa, delivering the active component to the body. Clinical investigations demonstrate that the active ingredients take effect in around 10 minutes, making it appropriate for emergency medicine, epilepsy treatments, and other pharmaceuticals.
ODTs are especially useful for patients who have trouble swallowing, such as geriatrics and pediatrics, because they require less liquid to dissolve the tablets. The platform can also disguise bitter flavors, making it useful for pediatric patients. Also, coated tablets can be transported. Furthermore, coated pills can be transferred to the patient’s colon, offering mucosa-adhering effects, which are especially beneficial for stomach-colon illnesses. The active components are combined with inorganic elements, including pure tricalcium phosphate microcapsules, to ensure minimal toxicological consequences.
Pharmaceutical Excipients Market Key Players:
- Ashland Global Holdings
- Archer Daniels Midland Company
- Evonik Industries AG
- BASF SE
- Croda International
- DuPont
- Associated British Foods
- Lubrizol Corporation
- Kerry Group
- Roquette Feres
Recent News of Pharmaceutical Excipients Market:
- In February 2024, Asahi Kasei, a healthcare firm based in the US, lowered the nitrate content in Pharmaceutical Excipient Ceolus. They launched Ceolus microcrystalline cellulose (MCC), having nitrite levels of 0.1 μg/g or less, to lower the possibility of potentially carcinogenic nitrosamine contaminants in medications and nutritional supplements. The company’s move comes after a considerable increase in public awareness of the health risks of nitrosamines in 2018, with the pharmaceutical sector doing extensive studies and research to determine the source of these impurities.
- In October 2023, Clariant, a specialty chemicals firm that focuses on sustainability, introduced three new VitiPure® excipients at CPHI Barcelona. These excipients allow for a variety of Active Pharmaceutical Ingredient (API) compositions and delivery routes, including sensitive ones such as mRNA vaccines and biologic pharmaceuticals. Clariant intends to become a one-stop solution supplier for the industry, capitalizing on its experience in pharmaceutical production. The new excipients answer the market’s expanding demand for high-purity components by overcoming the stability and bioavailability difficulties of Active Pharmaceutical components.
- In September 2023, Nitika Pharmaceuticals Specialties plans to open India’s largest Microcrystalline Cellulose production factory on September 16, 2023, with leaders Nitin Gadkari and Devendra Fadnavis in attendance. The plant, India’s only excipient manufacturer, will benefit from production-linked incentives, proving Nitika’s commitment to invention and strengthening India’s worldwide pharmaceutical position.
