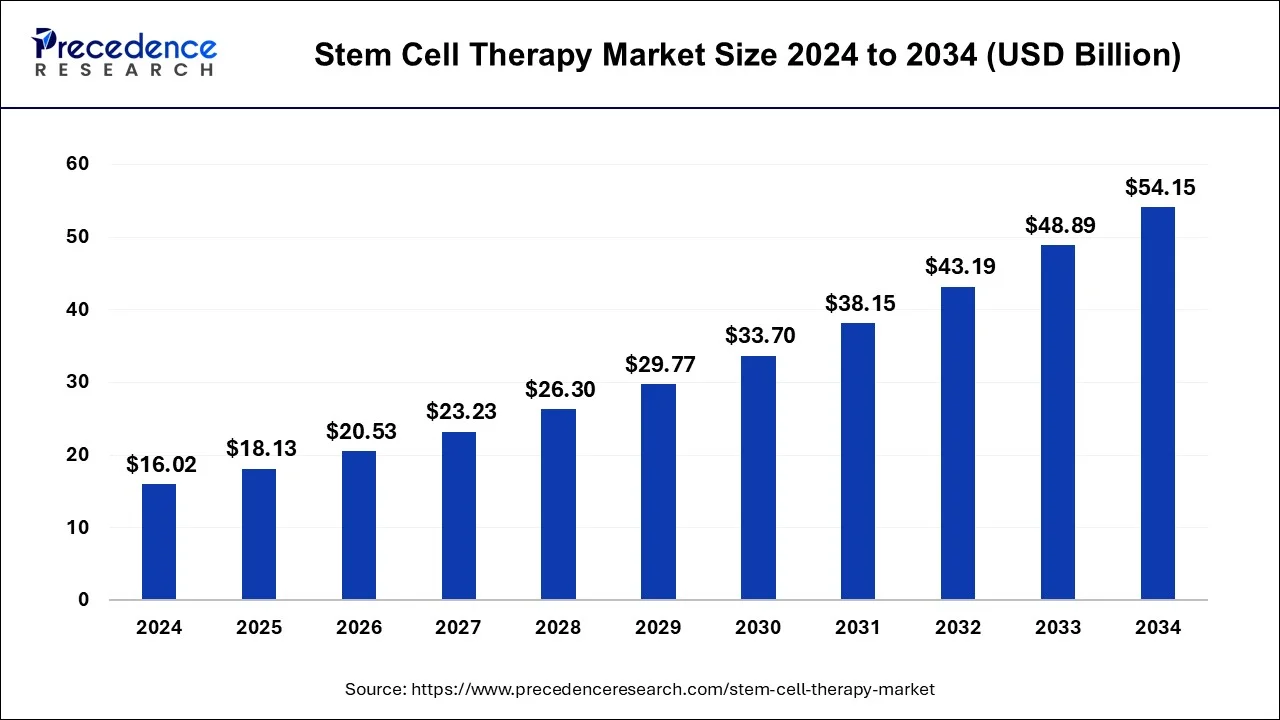
The global stem cell therapy market size was valued at USD 14.15 billion in 2023 and is expected to reach around USD 48.89 billion by 2033 with a CAGR of 13.2% from 2024 to 2033.
Market participants are directing resources toward oncology with an aim at extending cancer patients lives and discovering new treatments for autoimmune diseases, rheumatoid arthritis, heart diseases, spinal cord injuries, and diabetes. Since more research studies and clinical trials are being carried out, the stem cell therapy market is expected to continue growing. The rising number of diseases worldwide is likely to spur the demand for regenerative medicines, in which stem therapy is a key component. Moreover, the rising number of clinical trials further propels the market.
Key Insights
- North America has captured revenue share of around 54% in 2023.
- Asia Pacific is poised to grow at a CAGR of 10% from 2024 to 2033.
- By product, the adult stem cells (ASCs) segment has generated highest revenue share of 86.1% in 2023.
- The induced pluripotent stem cells (iPSCs) segment is expected to reach at a CAGR of 10% from 2024 to 2033.
- By application, the regenerative medicine segment has accounted revenue share of 93% in 2023.
- The drug discovery and development application segment is growing at a CAGR of 10.2% from 2024 to 2033.
- By therapy, allogenic stem cell therapy has held revenue share of around 59% in 2023.
Regional Stance
North America dominated the stem cell therapy market in 2023 due to the rising focus on personalized therapeutic approaches. Furthermore, rising investments in the research and development of regenerative medicines and the rising number of clinical trials bolstered the market in the region. The U.S. and Canada are the major contributors to the regional market growth due to their well-established healthcare systems and advanced research facilities. The regional market growth is further attributed to the favorable government policies to promote stem cell therapy.
The market in Asia Pacific is expected to grow at the fastest rate during the forecast period. This is mainly due to the increasing number of clinical trials, the rapid expansion of the biotechnology sector, and the growing prevalence of chronic diseases. Organizations, government programs, and intensified research activities can facilitate the demand for stem cell therapy in the region. The increasing medical tourism in emerging countries, such as Indi, China, Japan, and South Korea, and the rising government funding to expand healthcare infrastructure further fuel the market in Asia Pacific.
Report Highlights
Type Insights
The allogeneic segment dominated the global stem cell therapy market with the largest share in 2023. This is due to the greater scalability of allogeneic cell therapies compared to autologous therapies. Furthermore, allogeneic cell therapies use a single source of tissues to treat multiple patients. The key advantage of this therapy is the multiple potential cell sources, such as donated tissues, placenta, umbilical cord blood, bone marrow, and embryonic stem cells. These therapies can be derived from donors and utilizing them to treat various cancers. Therefore, allogeneic stem cell therapies offer quick access for several patients, making them suitable for off-the-shelf supply models. Furthermore, these treatments have greater scalability, thus leading to reduced production costs and less expense for patients.
The autologous segment is expected to grow at the fastest rate during the forecast period. By minimizing the potential for immune rejection and facilitating prolonged recovery, autologous cell therapies provide an innovative approach to treat cancer. This leads to fewer side effects and more chances of successful results. Autologous cell therapy also meets the specific needs of individual patients, thus making it possible to have highly targeted and effective treatments. With this personal method, using target cells to treat various tissues or organs could also translate into improved results and shortened healing times.
Application Insights
The regenerative medicine segment dominated the market in 2023. This is mainly due to the rising research and development activities for regenerative medicines. The government supports the development of these medicines to cure diabetes, heart diseases, and blood disorders, as well as chronic diseases. Regenerative medicine enables faster healing by allowing doctors to inject platelets or stem cells directly into an injured part of the body, thus concentrating healing energies. Regenerative treatments also help alleviate pain and inflammation naturally. These approaches promote overall health within the musculoskeletal system when new cells and highly concentrated platelets are infused into tissues and bones.
- According to the report published by the National Institute of Health, the number of companies providing services for regenerative medicine, including stem cell therapy, increased globally from 772 in 2016 to more than 1550 in 2024.
End User Insights
The hospitals segment dominated the market in 2023. This is due to the advanced infrastructure and different treatment options available in hospitals. The role of hospitals has been vital in developing novel therapies for various diseases and providing high-quality patient care through their collaborations with research labs. Such collaborations are crucial in developing specialized stem cell therapies.
Market Dynamics
Driver
Potential to treat range of disorders is fueling the market
The potential of stem cells to cure ailments and make healthy cells for regenerative medicine drives the market. Stem cells are also used to test the effectiveness and safety of drugs. Their capacity to regenerate and repair damaged tissues can help treat patients with Hodgkins disease, leukemia, non-Hodgkins lymphoma, aplastic anemia, solid tumor cancers, immunodeficiency, or inherited metabolic disorders. Moreover, stem cells are used in treating other diseases like Parkinsons disease, heart failure, Osteoarthritis, and type 1 diabetes. One of the emerging fields of study involves using human stem cells that are customized into specific tissue for accurate drug testing.
Restraint
Side effects associated with stem cell therapies
Stem cell-based therapies have not become commonplace in managing musculoskeletal disorders mainly due to concerns regarding tumorigenicity, ectopic tissue formation, and disease spreading. There may be side effects associated with strokes, such as destroying the blood-brain barrier, perilesional hyperexcitability, and systemic immunodepression. Exhaustion, nausea, hair loss, and vomiting are among the short-term side effects associated with stem cell therapy, while graft-versus-host disease, anemia, infection as well as bleeding are some of its long-term side effects. Furthermore, stem cell therapy can cause skin and hair problems, digestive system issues, and other possible complications, thus restraining the market.
Opportunity
Adoption of AI for increased efficiency
The use of AI to advance stem cell therapy creates immense opportunities in the market. Multiple sectors embrace AI to support stem cell therapy. Government institutions, private companies, and academic organizations increasingly incorporate AI to find solutions for using stem cells to cure diseases. However, AI has shown great potential in predicting the activities of stem cells. AI also enables real-time monitoring of patients undergoing stem cell therapy. For instance, the Chan Zuckerberg Initiative is developing a virtual cell that mimics the properties and behaviors of real cells to forecast how different cells react to external stimuli. It also focuses on developing AI-powered virtual cells to assist scientists in exploring the molecular underpinnings of human health and disease.
Recent Developments
- In April 2024, Memel Biotech launched a fully integrated advanced therapy development and manufacturing service at its facility in Klaipeda, Lithuania. The company aims to collaborate with both emerging and established biotech companies.
- In April 2024, OrganaBio launched HematoPAC-HSC-CB-GMP, ethically derived CD34+ hematopoietic stem cells from human cord blood. It utilizes its cell isolation and GMP manufacturing expertise for next-generation cell therapies.
- In April 2024, researchers at the University of California, San Francisco (UCSF) began enrollment for a clinical trial using a new CAR T version for glioblastoma, a common and deadly brain cancer, funded by a US$ 11 million grant from the California Institute for Regenerative Medicine, which funds stem cell and gene therapy research.
- In December 2023, Max Healthcare launched chimeric antigen receptor (CAR)-T cell therapy at its Delhi-NCR hospital.
Stem Cell Therapy Market Companies
- Caladrius
- ReNeuron Group plc
- CELGENE CORPORATION
- Virgin Health Bank
- STEMCELL Technologies Inc.
- Biovault family
- Opexa Therapeutics, Inc.
- Pluristem Therapeutics Inc
- Precious Cells International Ltd
- Mesoblast Ltd
- Seneca Biopharmaceuticals, Inc.
Segments covered in the report
By Product
- Adult Stem Cells (ASCs)
- Hematopoietic
- Mesenchymal
- Neural
- Epithelial/Skin
- Others
- Human Embryonic Stem Cells (HESCs)
- Induced Pluripotent Stem Cells (iPSCs)
- Very Small Embryonic Like Stem Cells
By Therapy Type
- Autologous
- Allogenic
By Application
- Regenerative Medicine
- Neurology
- Orthopedics
- Oncology
- Hematology
- Cardiovascular and Myocardial Infraction
- Injuries
- Diabetes
- Liver Disorder
- Incontinence
- Others
- Drug Discovery and Development
By Technology
- Cell Acquiition
- Bone Marrow Harvest
- Umbilical Blood Cord
- Apheresis
- Cell Production
- Therapeutic Cloning
- In-vitro Fertilization
- Cell Culture
- Isolation
- Cryopreservation
- Expansion and Sub-Culture
By End User
- Hospitals
- Research institutes
- surgical institutes
- Orders
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
Buy This Premium Research Report@https://www.precedenceresearch.com/checkout/1989
