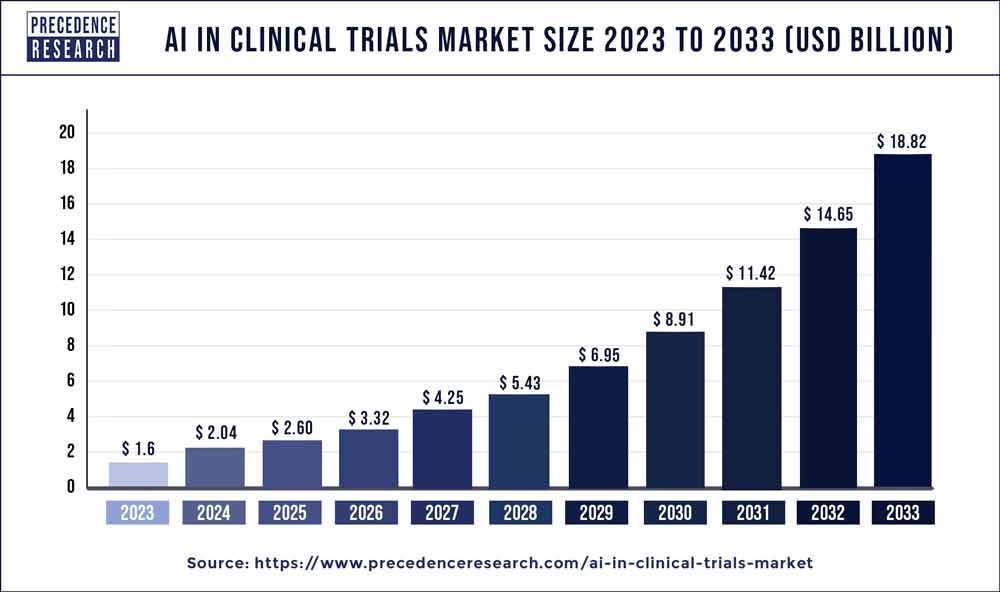
The global AI in clinical trials market size is projected to reach around USD 18.82 billion by 2033 from USD 1.6 billion in 2023 with significate a CAGR of 28% from 2024 to 2033.
The AI in Clinical Trials market is experiencing a paradigm shift propelled by the integration of cutting-edge technologies into the healthcare sector. Artificial Intelligence (AI) has emerged as a disruptive force in revolutionizing various aspects of clinical trials, offering enhanced efficiency, accuracy, and cost-effectiveness. Leveraging AI in clinical trials enables pharmaceutical companies, contract research organizations (CROs), and healthcare institutions to streamline processes, optimize decision-making, and accelerate the drug development timeline. With the increasing complexity of clinical research and the demand for innovative solutions, the AI in Clinical Trials market is poised for substantial growth in the coming years.
Key Points
- North America held the dominating share of the AI in clinical trials market in 2023.
- By offering, the services segment held the largest market share in 2023.
- By technology, the deep learning segment held the dominating market share in 2023.
- By application, the infectious disease segment dominated the market in 2023.
- By end-user, the pharmaceutical segment held the largest share of the market in 2023; the segment is observed to sustain dominance throughout the forecast period.
Growth Factors
Several factors contribute to the rapid growth of the AI in Clinical Trials market. Firstly, the escalating need to expedite drug discovery and development processes amidst rising healthcare challenges drives the adoption of AI-driven solutions. AI technologies such as machine learning, natural language processing (NLP), and predictive analytics empower researchers to analyze vast amounts of data efficiently, identify patterns, and make data-driven decisions swiftly. Additionally, the growing emphasis on personalized medicine and precision healthcare fuels the demand for AI-enabled tools that can enhance patient stratification, treatment selection, and clinical trial design. Furthermore, the proliferation of big data in healthcare, coupled with advancements in computational capabilities, facilitates the integration of AI into various stages of clinical trials, from patient recruitment to post-market surveillance.
AI in Clinical Trials Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 28% |
| Global Market Size in 2023 | USD 1.6 Billion |
| Global Market Size by 2033 | USD 18.82 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Offering, By Technology, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
AI in Clinical Trials Market Dynamics
Trends:
Several notable trends are shaping the landscape of AI in Clinical Trials. One prominent trend is the convergence of AI with other disruptive technologies such as blockchain and Internet of Things (IoT) to address data security, interoperability, and transparency challenges in clinical research. Moreover, there is a growing focus on decentralized clinical trials (DCTs) facilitated by AI-powered remote monitoring, wearable devices, and telemedicine solutions. This trend not only enhances patient participation and engagement but also reduces the burden of site visits and accelerates trial timelines. Furthermore, the integration of AI-driven virtual assistants and chatbots in patient recruitment and retention processes is gaining traction, enabling personalized interactions, real-time support, and data collection throughout the trial lifecycle.
Opportunities:
The AI in Clinical Trials market presents abundant opportunities for stakeholders across the healthcare ecosystem. Firstly, AI-driven platforms offer immense potential to optimize patient recruitment and retention strategies by identifying eligible participants more effectively and engaging them through personalized interventions. Moreover, AI-powered predictive analytics and risk modeling enhance the efficiency of clinical trial operations by forecasting patient outcomes, optimizing resource allocation, and mitigating potential risks. Furthermore, the advent of AI-driven biomarker discovery and diagnostic tools holds promise for advancing precision medicine initiatives and enabling targeted therapies for complex diseases. Additionally, the growing demand for real-world evidence (RWE) generation and post-market surveillance presents opportunities for AI-driven data analytics platforms to extract valuable insights from heterogeneous data sources and support evidence-based decision-making.
Restraints:
Despite its transformative potential, the adoption of AI in Clinical Trials faces certain challenges and constraints. One major restraint is the lack of regulatory clarity and standardized frameworks governing the use of AI-driven technologies in clinical research. Regulatory bodies are still grappling with issues related to data privacy, algorithm transparency, and validation of AI algorithms for clinical decision-making. Moreover, the integration of AI into existing clinical trial workflows requires substantial investments in infrastructure, training, and change management, which may pose barriers for smaller organizations with limited resources. Additionally, concerns regarding data quality, interoperability, and bias in AI algorithms underscore the importance of robust validation, transparency, and governance mechanisms to ensure the reliability and ethical use of AI in clinical trials.
Segments:
The AI in Clinical Trials market can be segmented based on various factors, including technology, application, end-user, and region. From a technological perspective, key segments include machine learning, natural language processing (NLP), predictive analytics, and computer vision. In terms of application, segments encompass patient recruitment and retention, clinical trial design and optimization, drug discovery and development, and post-market surveillance. End-user segments comprise pharmaceutical companies, contract research organizations (CROs), academic research institutes, and healthcare providers. Geographically, the market can be segmented into North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa, with North America accounting for a significant share due to the presence of established pharmaceutical companies, robust regulatory framework, and technological advancements.
Read Also: Network Engineering Services Market Size to Rise $97 Bn by 2033
Recent Developments
- In September 2023, a global pioneer in providing individuals and institutions with reliable intelligence to change the world, Clarivate Plc announced the creation of an Academia & Government Innovation Incubator. This will quicken its approach to fostering creativity, using AI, and launching cutting-edge products for its academic clients and users.
- In July 2023, by advancing the first medication identified and created by generative AI into Phase II clinical trials involving humans, Insilico Medicine has set a new standard in artificial intelligence drug research. The primary program, INS018_055, is a pan-fibrotic inhibitor that may be the first of its kind. Insilico’s moonshot medication unequivocally proves the viability of the company’s end-to-end AI drug development platform, Pharma. AI.
Competitive Landscape:
The competitive landscape of the AI in Clinical Trials market is characterized by intense competition, strategic partnerships, and continuous innovation. Key players operating in the market include pharmaceutical companies such as Pfizer Inc., Novartis AG, and Roche Holding AG, which are increasingly investing in AI-driven drug discovery and clinical trial optimization initiatives. Contract research organizations (CROs) such as IQVIA Holdings Inc., PAREXEL International Corporation, and ICON plc are leveraging AI technologies to enhance operational efficiency, data management, and patient recruitment services. Moreover, technology companies specializing in AI and healthcare analytics, such as IBM Watson Health, Microsoft Corporation, and Google Health, are developing advanced AI platforms tailored to the unique needs of clinical research. Furthermore, startups and emerging players focusing on niche AI applications, such as real-world data analytics, virtual clinical trials, and precision medicine, are gaining traction and disrupting the traditional market landscape. Overall, the competitive dynamics in the AI in Clinical Trials market are driven by innovation, scalability, and the ability to deliver tangible value to stakeholders across the drug development continuum.
AI in Clinical Trials Market Companies
- AiCure
- Antidote Technologies
- Deep 6 AI
- Mendel.ai
- Phesi
- Saama Technologies
- Signant Health
- Trials.ai
- Innoplexus
- IQVIA
- Median Technologies
- Medidata
Segments Covered in the Report
By Offering
- Software
- Services
By Technology
- Machine learning
- Deep learning
- Supervised
By Application
- Cardiovascular
- Metabolic
- Oncology
- Infectious diseases
By End-user
- Pharma
- Biotech
- CROs
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
