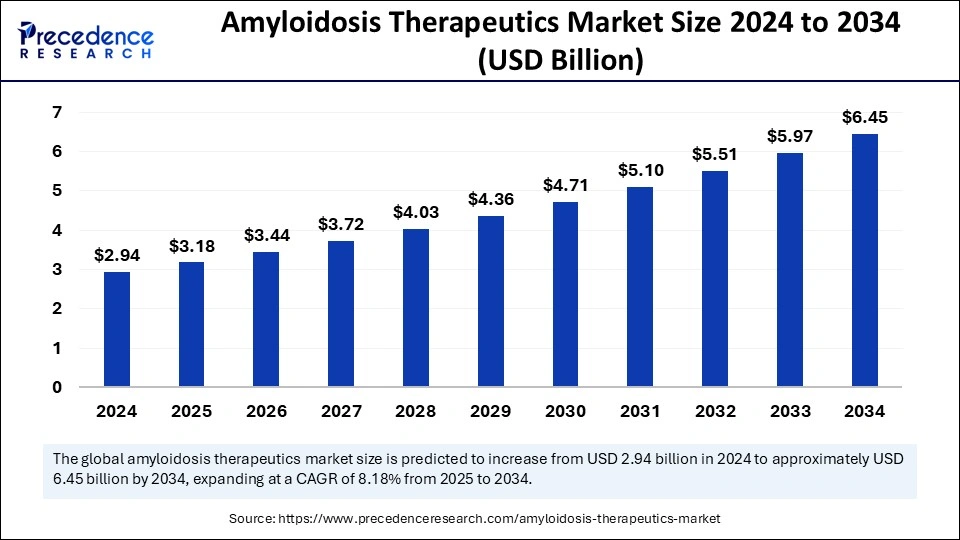
The Amyloidosis Therapeutics Market is set to increase from USD 2.94 billion in 2024 to around USD 6.45 billion by 2034, experiencing a CAGR of 8.18%.
Amyloidosis Therapeutics Market Critical Observations
- North America dominated the global market with the highest share of 47% in 2024.
- Asia Pacific is estimated to expand at the fastest CAGR in the market between 2025 and 2034.
- By treatment, the chemotherapy segment held the largest market share in 2024.
- By treatment, the transplantation segment is anticipated to grow at a remarkable CAGR between 2025 and 2034.
- By end user, the hospitals and clinics segment dominated the market with the largest share in 2024
- By end user, the home care settings segment is anticipated to show fastest growth during the predicted timeframe
The Amyloidosis Therapeutics Market is experiencing steady growth, driven by advancements in treatment options, increased awareness, and rising prevalence of amyloidosis globally. Amyloidosis is a rare but serious disease caused by the abnormal buildup of amyloid proteins in various organs, leading to organ dysfunction and failure. The growing understanding of different forms of amyloidosis, such as AL amyloidosis, ATTR amyloidosis, and AA amyloidosis, has prompted significant research and development in therapeutic approaches. Innovations in targeted therapies, monoclonal antibodies, and gene-editing technologies are expanding treatment possibilities, improving patient outcomes, and driving market growth.
The global market for amyloidosis therapeutics was valued at USD 2.94 billion in 2024 and is projected to reach approximately USD 6.45 billion by 2034, growing at a CAGR of 8.18% from 2025 to 2034. Increasing investments in biopharmaceutical research, improved diagnostic capabilities, and regulatory approvals for novel treatment options are fueling this expansion. Additionally, patient advocacy groups and government health initiatives are playing a crucial role in improving accessibility to treatments, further boosting market growth.
Sample Link: https://www.precedenceresearch.com/sample/5710
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 6.45 Billion |
| Market Size in 2025 | USD 3.18 Billion |
| Market Size in 2024 | USD 2.94 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.18% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Treatment, End User, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Core Factors
A key driver of the Amyloidosis Therapeutics Market is the rising prevalence of amyloidosis worldwide. While considered a rare disease, improved diagnostic techniques and awareness campaigns have led to an increase in reported cases. With an aging global population, the risk of developing amyloidosis-related complications has also escalated, further increasing demand for effective treatments.
The growing pipeline of targeted therapies and novel drugs is another major factor driving market expansion. Advances in RNA interference (RNAi) therapies, immunotherapy, and gene-based treatments are offering new hope to patients who previously had limited options. Monoclonal antibodies targeting amyloid fibrils, such as daratumumab and eprodisate, are showing promising results in clinical trials, providing better disease management and survival rates.
The expansion of clinical trials and regulatory support is significantly shaping the market landscape. Many pharmaceutical and biotechnology companies are actively investing in orphan drug development, benefiting from incentives such as fast-track approvals, extended market exclusivity, and financial grants. Regulatory agencies, including the FDA and EMA, are prioritizing treatments for rare diseases, expediting approval processes for promising amyloidosis drugs.
Potentials
The emergence of precision medicine and personalized treatment approaches presents a major opportunity for the Amyloidosis Therapeutics Market. With the ability to tailor treatments based on genetic profiles, the industry is moving toward more effective and patient-specific therapies. The application of biomarker-based diagnostics is also improving early detection, enabling timely intervention and better disease management.
The increasing adoption of RNA interference (RNAi) therapies is another promising area for market expansion. RNAi-based drugs, such as patisiran and vutrisiran, have demonstrated efficacy in reducing amyloid deposits, offering a breakthrough in amyloidosis treatment. These therapies, coupled with advancements in small interfering RNA (siRNA) technology, are paving the way for innovative solutions to combat the disease.
The rise in collaborations and partnerships among pharmaceutical companies, research institutions, and healthcare organizations is accelerating drug discovery and development. Major players are entering strategic alliances to develop and commercialize novel amyloidosis drugs, improving accessibility and affordability of treatments across different regions.
Obstacles
Despite its growth, the Amyloidosis Therapeutics Market faces several challenges, with high treatment costs being one of the most significant barriers. Many of the newly developed therapies, including RNA-based treatments and monoclonal antibodies, come with high price tags, making them inaccessible to a large portion of patients, especially in developing regions. Limited insurance coverage and reimbursement policies further exacerbate affordability issues.
The lack of disease awareness and misdiagnosis remains a persistent challenge in the market. Since amyloidosis shares symptoms with other diseases, it is often underdiagnosed or detected at an advanced stage, reducing the chances of effective treatment. Efforts to improve medical training, early screening programs, and public awareness initiatives are crucial to addressing this issue.
Another challenge is the complexity of drug development and regulatory hurdles. Due to the rarity of the disease, conducting large-scale clinical trials can be difficult, leading to prolonged drug approval timelines. Additionally, companies must meet stringent regulatory requirements for orphan drug designation, which can slow down market entry for new therapeutics.
Regional Insights
North America dominates the Amyloidosis Therapeutics Market, primarily due to strong healthcare infrastructure, increased research funding, and a high prevalence of amyloidosis cases. The United States leads in drug development, clinical trials, and regulatory approvals, with pharmaceutical giants focusing on innovative treatment strategies. The presence of patient advocacy organizations and financial support from government agencies further boosts market growth in the region.
Europe is another significant market, driven by advanced healthcare systems, increasing awareness programs, and supportive regulatory frameworks. Countries like Germany, France, and the United Kingdom are investing heavily in amyloidosis research, while government-backed health programs are facilitating faster drug approvals. The expansion of rare disease registries and genetic screening programs is also contributing to early diagnosis and better treatment outcomes.
The Asia-Pacific region is experiencing rapid growth due to improving healthcare accessibility, rising disposable incomes, and growing awareness of rare diseases. Countries like China, Japan, and India are witnessing increased investments in biotechnology research and orphan drug development. The expansion of clinical trials and government-led health initiatives is creating new opportunities for market penetration in the region. However, affordability and access to advanced treatments remain key challenges.
Latin America and the Middle East & Africa are emerging markets with growing potential. While awareness and diagnostic capabilities are still developing, rising healthcare expenditures, expanding pharmaceutical presence, and increased government support for rare disease treatments are expected to drive market growth. Efforts to enhance medical training and infrastructure will play a crucial role in improving access to amyloidosis therapies in these regions.
Don’t Miss Out: Solid Tumor Cancer Treatment Market
Market Key Players
- Alnylam Pharmaceuticals, Inc.
- Amgen Inc.
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- GSK plc.
- Takeda Pharmaceutical Company Limited
- Sanofi
- Pfizer Inc.
- Johnson & Johnson Services, Inc.
- Merck KGaA
- Novartis AG
- Ionis Pharmaceuticals
Recent Insights
The Amyloidosis Therapeutics Market has witnessed several groundbreaking developments in recent years. The approval and commercialization of RNAi-based therapies, such as patisiran (Onpattro) and vutrisiran (Amvuttra), have revolutionized treatment options for transthyretin-mediated amyloidosis (ATTR). These drugs have demonstrated significant efficacy in reducing amyloid deposits and improving patient quality of life.
Biopharmaceutical companies are actively investing in monoclonal antibody therapies, which aim to directly target amyloid fibrils and enhance clearance from affected organs. Several experimental drugs are in late-stage clinical trials, with promising results indicating improved survival rates and reduced disease progression.
The adoption of gene therapy and CRISPR-based technologies is gaining traction in amyloidosis research. Scientists are exploring the potential of gene-editing techniques to correct genetic mutations responsible for ATTR amyloidosis, offering a potential cure rather than symptom management. Although still in the early stages, these advancements hold significant promise for the future of amyloidosis treatment.
Strategic partnerships and acquisitions are shaping the competitive landscape, with major pharmaceutical firms collaborating to accelerate drug development and expand treatment portfolios. Companies are focusing on combination therapies and innovative drug delivery systems to enhance treatment effectiveness and patient compliance.
Market Segmentation
By Treatment
- Chemotherapy
- Immunosuppressive Drugs
- Transplantation
- Supportive Care
- Surgery
- Others
By End User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Home Care Settings
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
