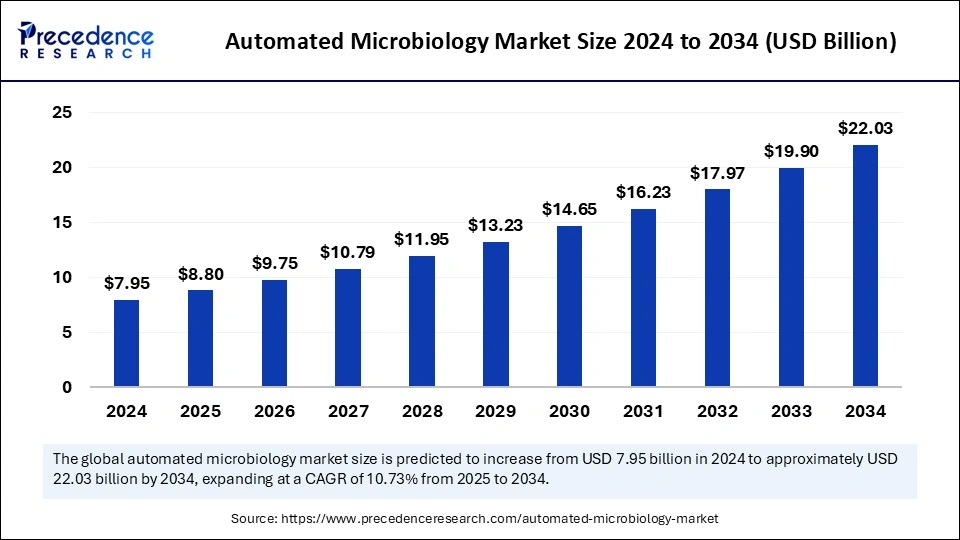
The automated microbiology market is forecasted to rise from USD 7.95 billion in 2024 to nearly USD 22.03 billion by 2034, reflecting a CAGR of 10.73%.
Automated Microbiology Market Critical Observations
- North America led the market by holding more than 41% of the market share in 2024.
- Asia Pacific is expected to grow at the fastest CAGR over the studied period.
- By product, the reagents and kits segment held the biggest market share of 49% in 2024.
- By product, the instruments segment is anticipated to grow at the fastest CAGR over the forecast period.
- By automation type, the fully automated segment led the market in 2024.
- By application, the clinical diagnostics segment dominated the market in 2024.
- By application, the biopharmaceutical production segment is anticipated to grow at the fastest CAGR over the forecast period.
- By end use, the hospitals and diagnostic laboratories segment held the biggest market share of 45% in 2024.
- By end use, the pharmaceutical and biotechnology companies segment is anticipated to grow at the fastest rate during the projected period.
The automated microbiology market is experiencing robust growth due to the increasing demand for faster, more accurate diagnostic solutions in healthcare, pharmaceuticals, and food safety. Automation in microbiology has transformed laboratory workflows, significantly improving efficiency, reducing human error, and enabling high-throughput sample analysis. The adoption of automated microbiology systems is driven by advancements in artificial intelligence, robotics, and molecular diagnostics, making them indispensable in clinical diagnostics, research, and industrial microbiology.
The rising prevalence of infectious diseases and antimicrobial resistance has heightened the need for rapid and precise microbial identification, pushing laboratories and healthcare facilities toward automation. Traditional microbiology methods are often labor-intensive and time-consuming, whereas automated systems provide quicker results, leading to faster treatment decisions and improved patient outcomes. The growing investment in healthcare infrastructure and increasing awareness of early disease detection have further propelled the market’s expansion.
With continuous technological innovations, automated microbiology is being integrated with data analytics and cloud-based solutions, enhancing workflow management and result interpretation. The rising trend of personalized medicine and genomic sequencing also supports the growth of automated microbiology, as these technologies require sophisticated microbiological analysis. As laboratories seek to improve efficiency and comply with stringent regulatory standards, the demand for automation in microbiology is expected to rise steadily.
Sample Link: https://www.precedenceresearch.com/sample/5722
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 22.03 Billion |
| Market Size in 2025 | USD 8.80 Billion |
| Market Size in 2024 | USD 7.95 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 10.73% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Automation Type, Application, End use, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East |
Core Factors
The growing burden of infectious diseases worldwide has been a significant driver of the automated microbiology market. The need for rapid pathogen detection, particularly in hospital settings, has led to a surge in demand for advanced diagnostic tools. Automated microbiology systems enable quicker turnaround times, aiding in the timely administration of appropriate treatments and reducing hospital stays. The rise in antimicrobial resistance has also necessitated the adoption of precise diagnostic methods to identify drug-resistant strains and guide effective treatment strategies.
The pharmaceutical and biotechnology industries are increasingly relying on automated microbiology for drug development and quality control. Ensuring microbial safety in biopharmaceutical production is critical, and automation allows for standardized, reproducible testing with minimal human intervention. Additionally, stringent regulatory requirements in pharmaceutical microbiology have encouraged the adoption of automated systems to meet compliance standards.
Food and beverage manufacturers are also contributing to market growth, as foodborne illnesses remain a global concern. Automated microbiology is widely used in food safety testing to detect pathogens and contaminants, ensuring compliance with safety regulations. With increasing consumer awareness and regulatory scrutiny on food safety, manufacturers are integrating automation to maintain high-quality standards and minimize the risk of contamination.
Potentials
The expansion of microbiological research and clinical diagnostics in emerging markets presents significant growth opportunities for the automated microbiology market. Developing countries are investing heavily in healthcare infrastructure, leading to increased demand for advanced laboratory technologies. Governments and private organizations are supporting initiatives to enhance disease surveillance and outbreak control, further driving the adoption of automated microbiology systems.
Technological advancements in artificial intelligence and machine learning are opening new possibilities for automated microbiology. AI-powered systems can analyze large datasets, detect patterns, and provide predictive insights, improving diagnostic accuracy and efficiency. The integration of AI with microbiology automation has the potential to revolutionize laboratory workflows by reducing manual intervention and enhancing decision-making capabilities.
The increasing adoption of cloud-based laboratory information management systems (LIMS) is another key opportunity. Automated microbiology systems are being integrated with cloud platforms, allowing real-time data access, remote monitoring, and improved collaboration between laboratories and healthcare providers. This connectivity enhances operational efficiency and facilitates better disease tracking and epidemiological studies.
Obstacles
Despite its advantages, the adoption of automated microbiology systems comes with challenges. High initial investment costs can be a barrier, particularly for small and medium-sized laboratories. The cost of purchasing, installing, and maintaining advanced automation systems may deter budget-constrained facilities from transitioning to automated microbiology.
Technical complexities and the need for skilled professionals to operate and manage automated microbiology systems pose another challenge. While automation reduces manual workload, it requires specialized training to ensure accurate calibration, maintenance, and result interpretation. The shortage of trained microbiologists and laboratory technicians in certain regions may hinder the widespread adoption of automation.
Regulatory compliance remains a critical challenge for automated microbiology manufacturers. Stringent guidelines governing clinical diagnostics, pharmaceutical microbiology, and food safety testing require companies to meet rigorous validation and certification processes. Ensuring that automated systems comply with regional and international standards can be time-consuming and costly for manufacturers.
Regional Insights
North America dominates the automated microbiology market, driven by the presence of advanced healthcare infrastructure, a high prevalence of infectious diseases, and strong research and development activities. The U.S. leads the region with significant investments in clinical diagnostics and pharmaceutical microbiology. Government initiatives promoting disease surveillance and infection control have further contributed to market growth.
Europe is another major market, with a strong focus on laboratory automation in healthcare and pharmaceutical industries. Countries like Germany, the U.K., and France are at the forefront of adopting automated microbiology solutions to enhance diagnostic accuracy and efficiency. The European Union’s stringent regulations on food safety and drug quality have accelerated the integration of automation in microbiological testing.
Asia-Pacific region is witnessing rapid growth, fueled by increasing healthcare expenditure, rising awareness of infectious diseases, and expanding pharmaceutical and food industries. Countries like China, Japan, and India are investing heavily in modernizing healthcare infrastructure, leading to higher demand for automated microbiology systems. The growing biopharmaceutical sector in the region further supports market expansion.
Latin America and the Middle East & Africa are gradually embracing automation in microbiology, particularly in clinical diagnostics and food safety testing. While these regions face economic constraints, improving healthcare facilities and regulatory reforms are expected to drive market growth. The increasing focus on controlling infectious disease outbreaks and enhancing laboratory capabilities presents future opportunities for expansion.
Don’t Miss Out: Solid Tumor Cancer Treatment Market
Industry Leaders
- BD
- QIAGEN
- Thermo Fisher Scientific Inc.
- Agilent Technologies, Inc.
- Danaher
- Merck KGaA
- bioMérieux
- Abbott
- DiaSorin S.p.A.
- BioRad Laboratories, Inc.
Recent Insights
Technological advancements continue to shape the automated microbiology market, with major players introducing next-generation systems that enhance speed, accuracy, and ease of use. Recent developments include AI-driven diagnostic platforms that integrate machine learning algorithms to improve microbial identification and resistance profiling. These innovations are streamlining microbiological testing and enabling more precise disease management.
Pharmaceutical companies are increasingly collaborating with automated microbiology firms to enhance drug development processes. Automation in microbiology is being leveraged to improve sterility testing, environmental monitoring, and bioprocess quality control, ensuring compliance with stringent regulatory standards. The rise of biologics and personalized medicine has further emphasized the need for high-throughput, automated microbiological testing solutions.
The food industry is witnessing increased adoption of automated microbiology for rapid pathogen detection and quality assurance. Recent regulatory updates in food safety have pushed manufacturers to integrate advanced testing technologies to prevent contamination and ensure compliance with global safety standards. The demand for real-time microbial monitoring in food processing and packaging is driving further innovation in this sector.
The COVID-19 pandemic highlighted the importance of automation in microbiology, with laboratories worldwide adopting automated systems to enhance testing capacity and speed. The experience gained during the pandemic has reinforced the value of automation in handling large-scale diagnostic challenges, setting the stage for continued investment in microbiology automation solutions.
With ongoing technological advancements, increasing investments in healthcare and food safety, and the continuous evolution of regulatory landscapes, the automated microbiology market is poised for steady growth in the coming years. The integration of AI, IoT, and cloud-based solutions will further transform microbiological testing, making it more efficient, accessible, and impactful across various industries.
Market Segmentation
By Product
- Instruments
- Automated Microbial Identification Systems
- Automated Blood Culture Systems
- Automated Colony Counters
- Automated Sample Preparation Systems
- Automated Antibiotic Susceptibility Testing (AST) Systems
- Other Instruments
- Reagents and Kits
- Culture Media
- Stains and Dyes
- Assay Kits and Panels
- Others
- Software
By Automation Type
- Fully Automated
- Semi-Automated
By Application
- Biopharmaceutical Production
- Clinical Diagnostics
- Environmental and Water Testing
- Food and Beverage Testing
- Other Applications
By End-use
- Pharmaceutical and Biotechnology Companies
- Hospitals and Diagnostic Laboratories
- Academic and Research Institutes
- Other End Use
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
