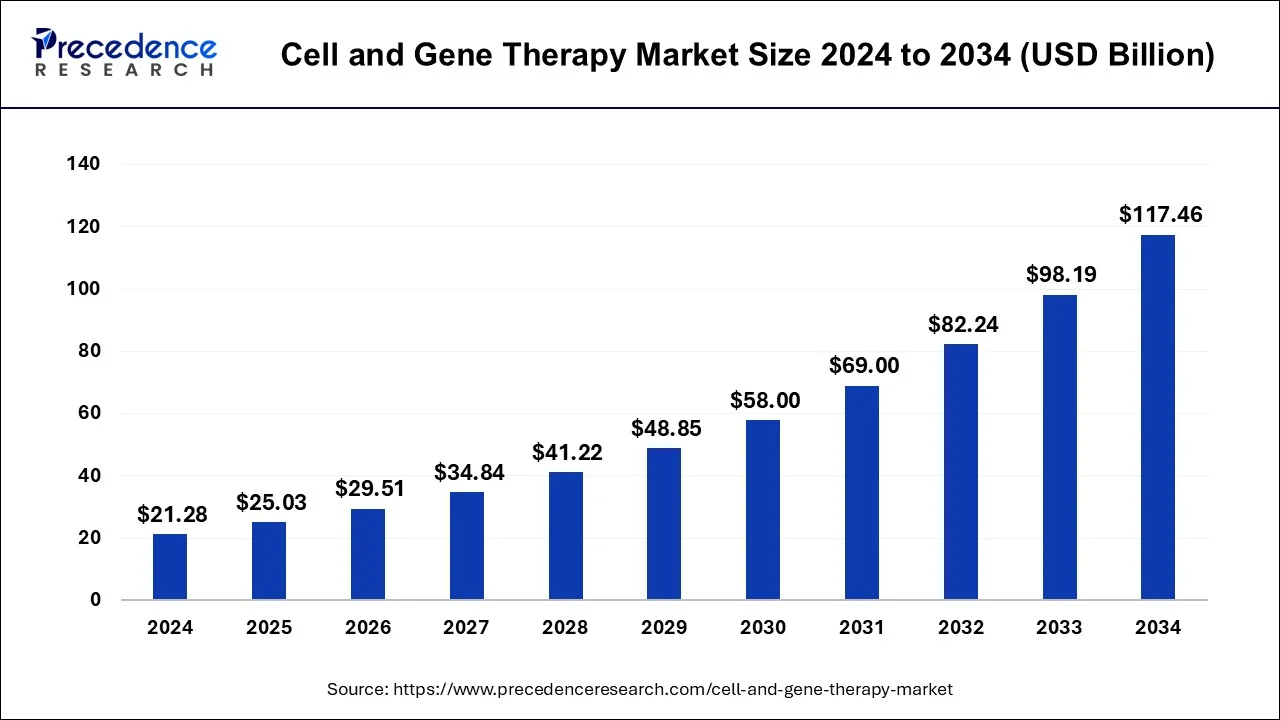
The cell and gene therapy market size surpassed USD 18.12 billion in 2023 and is anticipated to cross around USD 117.46 billion by 2034 with a remarkable CAGR of 18.6% from 2024 to 2034.
Cell and Gene Therapy Market Overview
The cell and gene therapy market refers to a sector within the biopharmaceutical industry that focuses on developing, producing, and commercializing treatments for the cure or treatment of diseases by manipulating genetic material or living cells. Cell and gene therapy are overlapping areas of biological study and treatment that treat, prevent, or cure diseases. Both therapies help to abolish the primary causes of hereditary and acquired disorders.
Cell therapy involves replacing or modifying specific cells in a body. In contrast, gene therapy involves introducing genes into a cell, inactivating a gene, or replacing a gene with a functional copy inside the cell, either in vivo or ex vivo. Some drugs act as both cell and gene therapies, as their mechanisms of action involve modifying genes in specific cell types and their subsequent delivery into the body.
Get a Sample: https://www.precedenceresearch.com/sample/2445
Cell and Gene Therapy Market Key Highlights:
- North America dominated the cell and gene therapy market with the largest market share 49.70% in 2023.
- By therapy, the cell therapy segment contributed more than 86.19% of the market share in 2023.
- By therapeutic, the infectious disease segment accounted for the largest market share of 28.73% in 2023.
- By delivery method, the in vivo segment generated more than 79.19% of the market share in 2023.
- By end-users, the cancer care centers segment has captured the biggest market share of 40.20% in 2023.
Cell and Gene Therapy Market Revenue (USD Million), By Therapy Type, 2020-2023
| By Therapy Type | 2020 | 2021 | 2022 | 2023 |
| Cell Therapy | 7,039.46 | 11,509.23 | 13,396.01 | 15,621.48 |
| Gene Therapy | 1,005.64 | 1,710.27 | 2,067.97 | 2,502.14 |
Cell and Gene Therapy Market Revenue (USD Million), By Delivery Method, 2020-2023
| By Delivery Method | 2020 | 2021 | 2022 | 2023 |
| In Vivo | 6,333.10 | 10,427.20 | 12,221.99 | 14,352.68 |
| Ex Vivo | 1,712.00 | 2,792.30 | 3,241.99 | 3,770.93 |
Cell and Gene Therapy Market Analysis by Geography
North America dominated the cell and gene therapy market in 2023 owing to continued research in gene therapy. However, various ethical and safety protocols limit the market expansion. The increasing need for quality healthcare for cancers and genetic diseases and the rising development of improved therapy and diagnostic techniques boost the market.
- For instance, in January 2024, the U.S. FDA announced the launch of its pilot program called Collaboration on Gene Therapies Global Pilot (CoGenT Global). Under the program, the Center for Oncology Excellence is collaborating with the World Health Organization and the International Council for Harmonisation through Project Orbis to discuss the potential for concurrent and collaborative review of gene therapy applications.
- In April 2024, the FDA approved Pfizer’s first gene therapy for the rare genetic bleeding disorder. The agency cleared the drug, to be sold under the brand name Hemgenix, for the treatment of adults with moderate to severe hemophilia B who require factor IX replacement therapy, as indicated. A Pfizer spokesperson confirmed to CNBC that eligible patients will be able to get the treatment this quarter by prescription. It comes with a whopping price tag of US$ 3.5 million before insurance and other rebates.
Cell and Gene Therapy Market Segmental Analysis
Analysis by Therapy
The cell therapy segment dominated the cell and gene therapy market in 2023. Cell therapy involves transplanting human cells to replace damaged tissue or repair tissues. With the advancement in technology, several types of cells can be used for several diseases and conditions.
Hematopoietic stem cells (HSCs) are used to treat blood cancers and hematologic conditions. Other potential applications are being researched for cancer, autoimmune, urinary problems, infectious diseases, repairing joint damage, repairing spinal cord damage, improving the immune system, and neurologic disorders.
Analysis by Therapeutic
The infectious disease segment dominated the market in 2023. Since the emergence of contagious agents such as HIV, various pharmaceutical compounds have been studied for treatment. However, these therapies have yet to be promising in curing SARS-CoV-2-associated lung injury.
Stem cell treatment, a new method with immunomodulatory effects on covering cells and inducing antioxidants, is being researched to restore damaged lungs and resolve the inflammatory response. Stem cells, which have CD marks and diminish the proinflammatory cytokines, can increase rapidly and are considered a well-recognized solution for cellular treatments.
Read Also: Battery Market Size Expected to Attain USD 680.85 Bn by 2034
Analysis by Delivery Mode
The in vivo therapy segment is anticipated to grow significantly during the projected period.
In vivo gene therapy involves coating a normal F8 gene through a viral vector and administering it into the body via intravenous infusion. This technique targets inside organs; no cells are removed from the body, so it is relatively easier. However, the genetic material should be delivered exactly at the injury site, which may trigger an immunological response if not properly done.
Cell and Gene Therapy Market Dynamics
Driver:
Rising prevalence of chronic diseases
The cell and gene therapy market is growing rapidly due to the growing prevalence of chronic disorders. Cancer is the leading cause of death in the U.S. and across the globe. The cost of cancer treatment in the U.S. was more than US$ 180 billion in 2015 and is estimated to reach US$ 246 billion by 2030.
Lung cancer is the leading cause of cancer death worldwide, followed by colorectal cancer. The cancer incidence rate is expected to rise by more than 40% by 2040, with the global cost of cancer likely to exceed US$ 25 trillion between the years 2020 and 2050.
Aging people
The world’s population is on the rise, and by 2030, one in every six people will be in their sixties or older. This aging trend, otherwise called the process of population aging, initially started in countries with a high-income economy but is very fast spilling over into countries with low- and middle-income earnings.
By 2050, these countries will have two-thirds of the world’s over-60 population. Aging is defined as the accumulation of molecular and cellular damage over time, which leads to the loss of physical and mental functionality, vulnerability to various pathologies, and, eventually, death. Ageing also lead to death.
Restraint:
Targeting correct cell for therapy
Gene therapy cures some diseases by inserting one normal gene into the correct cells in the tissue. However, inserting that gene into the wrong cells will bring about inefficiency and yield severe health disorders. This mistargeting allows the therapeutic gene to enter the patient’s germline and could possibly be transmitted from the patient to his children.
Opportunity: Evolving novel technologies
Advanced solutions are being developed to support the GMP manufacturing step of cell therapy, increasing speed and cost-effectiveness and opening the opportunity for cell and gene therapy market growth. These methods are also used to create novel cell therapy products for common ailments such as cancer and immune deficiency disorders.
These technologies can also work in areas where handling highly concentrated and complex recombinant biologic formulations poses challenges, including controlled release and injectability. New technology solutions are being created to better adoptive cell therapies such as CAR-T, TCR, and TIL by bringing down their prices, resolving rejection issues, guiding the CAR to act at a specific region, and generating new strategies for immunotherapies.
Cell and Gene Therapy Market Companies
- Alnylam Pharmaceuticals Inc.
- Amgen Inc.
- Biogen Inc.
- CORESTEM Inc.
- Dendreon Pharmaceuticals LLC.
- Helixmith Co. Ltd.
- JCR Pharmaceuticals Co. Ltd.
- Kolon TissueGene Inc.
- Novartis AG
- Pfizer Inc.
Recent Developments
- McKinsey will launch a new Digital Capability Center to quicken the development of cell and gene therapies (CGT). The Digital Capability Center will focus on accelerating operational excellence and digital transformations in biopharmaceutical manufacturing. The Digital Capability Center will join McKinsey’s rapidly growing global network of Digital Capability Centers, immersive learning environments that inspire and prepare organizations to deliver sustainable performance improvement from operational-excellence and tech-enabled transformations.
- on october 3, 2022, Alexion’s genomic medicines annuoced the acquisition of LogicBio’s technology, by this acquiestuion they are looking to incorpate knowledgeable team for preclinical development, and team for research and development on rare diseases. LogicBio has developed a number of technical systems for the delivery and insertion of genes to address genetic disorders. Additionally, they will built a platform to improve the creation of viral vectors.
- On October 12, 2022, Moderna disclosed that Merck(MSD) has decided to exercise its $250 million option to co-develop and commercialize PCV mRNA-4157/V940. The vaccine is now being tested in a phase II clinical trial as adjuvant therapy for patients with high-risk melanoma in combination with pembrolizumab, Merck’s programmed cell death protein 1 (PD-1) antibody.
- On October 3, 2022, Pfizer announced that they have successfully purchased Biohaven Pharmaceuticals which is a migraine drug manufacturing company. They recently manufactured NURTEC ODT (rimegepant) which is approved for both acute therapy and episodic migraine prevention in adults. With Pfizer’s global reach and this acquisition, they will be able to provide migraine patients with more treatment alternatives.
Segments Covered in the Report
By Therapy Type
- Cell Therapy
- Stem Cells
- T Cells
- Dendritic Cells
- NK Cells
- Tumor Cells
- Gene Therapy
By Therapeutic class
- Cardiovascular Disease
- Cancer
- Genetic Disorder
- Rare Diseases
- Oncology
- Hematology
- Ophthalmology
- Infectious Disease
- Neurological Disorders
- Others
By Delivery Method
- In Vivo
- Ex Vivo
By End-Users
- Hospitals
- Cancer Care Centers
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- MEA
