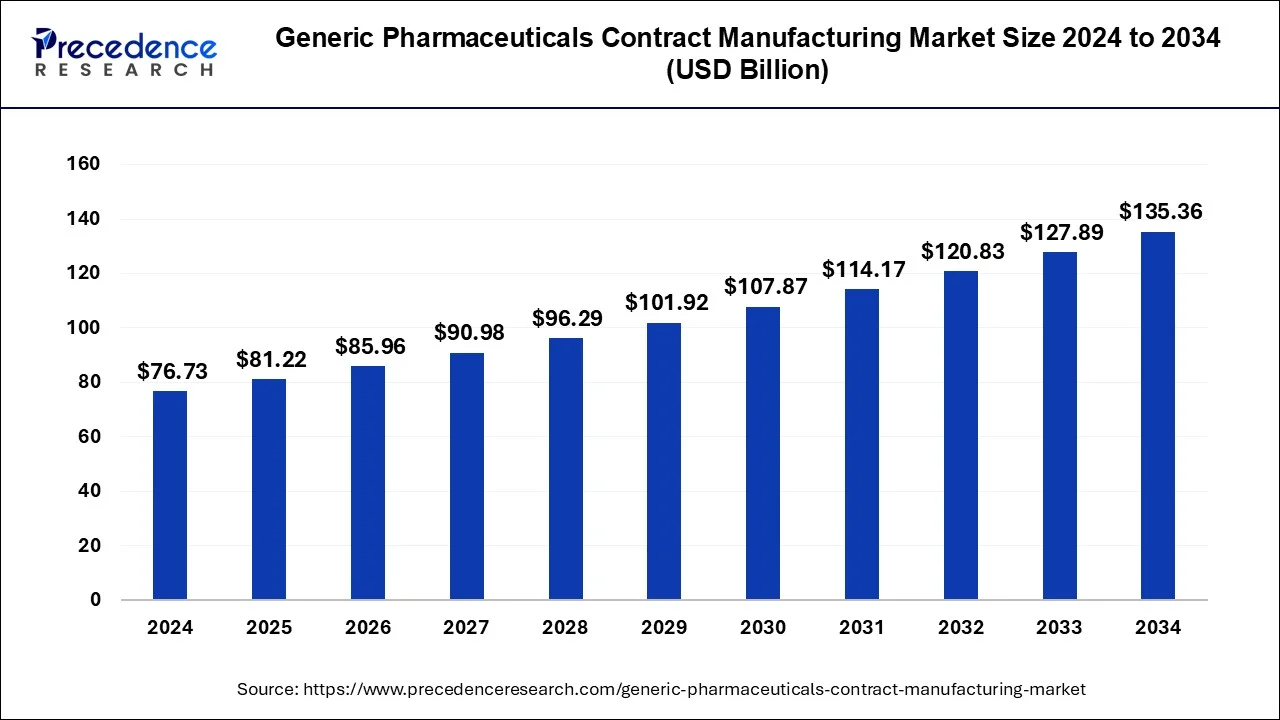
The global generic pharmaceuticals contract manufacturing market size reached USD 72.50 billion in 2023 and is projected to surpass around USD 135.36 billion by 2034, expanding at a CAGR of 5.84% from 2024 to 2034.
The Generic Pharmaceuticals Contract Manufacturing Market plays a pivotal role in the pharmaceutical industry by offering cost-effective manufacturing solutions for generic drugs. This segment has witnessed significant growth due to the increasing demand for affordable medicines globally. Contract manufacturing organizations (CMOs) in this sector specialize in producing pharmaceuticals that are bioequivalent to brand-name drugs but at lower costs, facilitating broader access to essential medications.
Generic Pharmaceuticals Contract Manufacturing Market Key Takeaways
- North America is estimated to be the fastest-growing during the forecast period of 2024-2033.
- By drug type, the branded generics segment has contributed more than 63% of revenue share in 2023.
- By drug type, the unbranded generics segment is significantly growing during the forecast period.
- By product, the API product segment has held a major revenue share of 58% in 2023.
- By route of administration, the oral segment has captured the largest revenue share of 62% in 2023.
- By route of administration, the parenteral segment is anticipated to be the fastest-growing during the forecast period.
- By application, the oncology segment has generated the biggest revenue share of 23% in 2023.
- By application, the immunology segment is expected to be the fastest-growing during the forecast period.
Get a Sample: https://www.precedenceresearch.com/sample/4674
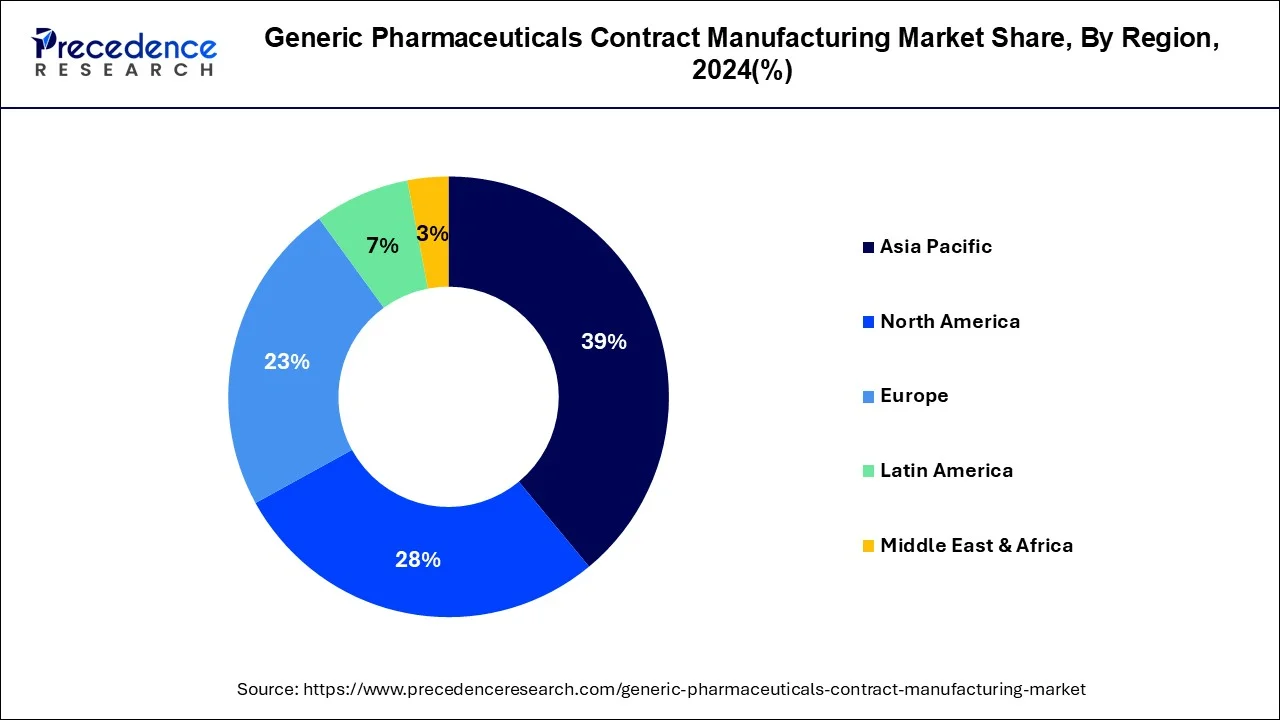
Regional Insights
Facial Recognition Market Trends
- Increased Demand for Cost-Effective Medications: With rising healthcare costs globally, there is a growing demand for affordable generic drugs. Contract manufacturers play a crucial role in producing these generics at lower costs through economies of scale and efficient manufacturing processes.
- Growing Outsourcing of Manufacturing Activities: Pharmaceutical companies are increasingly outsourcing their manufacturing operations to contract manufacturers to focus on core competencies such as research, development, and marketing. This trend allows for flexibility in production capacity and geographic reach.
- Advancements in Manufacturing Technologies: Technological advancements in pharmaceutical manufacturing, such as continuous manufacturing and automation, are being adopted by contract manufacturers. These technologies improve efficiency, reduce production costs, and enhance product quality and consistency.
- Increasing Regulatory Scrutiny and Compliance: Stringent regulatory requirements and quality standards drive pharmaceutical companies to partner with contract manufacturers that adhere to Good Manufacturing Practices (GMP) and other regulatory guidelines. Compliance with global standards is crucial for market acceptance and expansion.
- Focus on Specialty and Complex Generic Drugs: There is a shift towards manufacturing complex generics and specialty drugs, which require specialized capabilities and expertise. Contract manufacturers with experience in handling complex formulations and processes are in high demand.
Generic Pharmaceuticals Contract Manufacturing Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 135.36 Billion |
| Market Size in 2023 | USD 72.50 Billion |
| Market Size in 2024 | USD 76.73 Billion |
| Market Growth Rate from 2024 to 2034 | CAGR of 5.84% |
| Largest Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Drug, Product, Route of Administration, Application, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Drug Insights
The Generic Pharmaceuticals Contract Manufacturing Market encompasses a wide range of drug types tailored to meet diverse healthcare needs. This includes the production of generic drugs, which are identical or bioequivalent to brand-name medications after their patent expiry. Specialty generics are also prominent, involving complex formulations that require specialized manufacturing capabilities. Additionally, the market includes biosimilars, which are generic versions of biologic drugs, demanding advanced biotechnological processes for production. Over-the-counter (OTC) drugs, offering non-prescription medications, and niche pharmaceuticals catering to rare diseases further diversify the market’s drug portfolio.
Product Insights
Within the Generic Pharmaceuticals Contract Manufacturing Market, a variety of products are manufactured to cater to different therapeutic requirements. Solid oral dosage forms like tablets and capsules are prevalent, offering convenient administration and precise dosing for patients across various treatment regimes. Injectables, including sterile solutions for intravenous, intramuscular, or subcutaneous delivery, address critical therapeutic needs requiring precise formulation and stringent quality control. Topicals such as creams, ointments, gels, and lotions provide localized treatment options for dermatological and mucosal conditions. Liquid oral formulations, powders for reconstitution or inhalation, and suppositories for rectal or vaginal use further expand the market’s product offerings.
Route of Administration Insights
The route of administration plays a crucial role in drug delivery methods within the Generic Pharmaceuticals Contract Manufacturing Market. Oral formulations remain a cornerstone, facilitating easy ingestion through tablets, capsules, and syrups, suitable for a wide range of therapeutic applications. Parenteral routes, including intravenous, intramuscular, and subcutaneous injections, ensure rapid and direct delivery of medications into the bloodstream or tissues, crucial for critical care and emergency treatments. Topical applications provide localized treatment options, offering relief for skin disorders and mucosal conditions. Inhalation routes deliver medications directly to the lungs, beneficial for respiratory therapies, while rectal and vaginal suppositories offer specific localized treatment options for gastrointestinal and gynecological conditions.
Application Insights
Applications within the Generic Pharmaceuticals Contract Manufacturing Market cover a broad spectrum of therapeutic areas and healthcare specialties. Cardiovascular medications address heart and circulatory system disorders, while central nervous system (CNS) drugs manage neurological and psychiatric conditions. Oncology treatments target various forms of cancer, employing specialized therapies to combat tumor growth and spread. Endocrinology drugs manage hormonal imbalances and metabolic disorders, supporting diabetes management and thyroid conditions. Gastroenterology medications address digestive system disorders, providing relief from conditions affecting the stomach, intestines, and liver. Respiratory medications treat disorders affecting the lungs and airways, including asthma and chronic obstructive pulmonary disease (COPD). Dermatology products manage skin conditions, including eczema and acne, while infectious disease treatments combat bacterial, viral, and fungal infections. Ophthalmology medications cater to eye health, treating conditions like glaucoma and infections. Urology medications manage disorders of the urinary tract and reproductive system, supporting kidney health and prostate conditions. Women’s health products address gynecological conditions and reproductive health, including contraception and hormone replacement therapies. Pediatrics medications are formulated specifically for children’s healthcare needs, ensuring safe and effective treatment options for young patients. Nutraceuticals provide nutritional supplements and dietary aids, promoting overall health and wellness. Veterinary pharmaceuticals address animal health needs, including medications for companion animals and livestock.
Opportunities
Opportunities in the generic pharmaceuticals contract manufacturing market abound, driven primarily by the rising prevalence of chronic diseases and the need for cost containment in healthcare expenditures. CMOs capitalize on economies of scale and operational efficiencies to offer competitive pricing, making generic drugs a preferred choice for healthcare providers, insurers, and patients alike. Moreover, partnerships between pharmaceutical companies and CMOs enable swift market entry for new generics, leveraging regulatory expertise and manufacturing capabilities.
Challenges
However, the market faces several challenges that impact its dynamics. Quality control and regulatory compliance are critical concerns, as CMOs must adhere to stringent global standards to ensure the safety and efficacy of produced drugs. Managing intellectual property rights and navigating complex supply chains can also pose challenges, particularly in global markets with varying regulatory requirements. Furthermore, fluctuations in raw material costs and the need for continuous technological upgrades add complexity to maintaining competitive pricing while ensuring profitability.
Read Also: Facial Recognition Market Size to Surpass USD 32.53 Bn by 2034
Generic Pharmaceuticals Contract Manufacturing Market Companies
- Metrics Contract Services
- Curia Global, Inc.
- Pfizer Centre One
- Syngene International Ltd.
- Acme Generics Pvt Ltd.
- Catalent, Inc.
- Alcami Corp., Inc.
- Cambrex Corp.
- Aurobindo Pharma
- Siegfried Holding AG
- Recipharm AB
- Jubilant Generics Ltd.
- Metrics Contract Services
Recent Developments
- In July 2023, Breyna Inhalation Aerosol, the first generic version of AstraZeneca’s Symbicort with an ANDA (abbreviated new drug application), was approved by the U.S. FDA (Food & Drug Administration) and launched by a global health company Viatris Inc. and Kindeva Drug Delivery L.P. for people with chronic obstructive pulmonary disease and asthma.
- In November 2023, an antibiotic manufacturing facility in Kundl, Austria was opened by a generic pharmaceuticals company based in Switzerland, Sandoz Group AG. In Europe, to strengthen the future of antibiotics manufacturing Sandoz invested $160.4m.
- In February 2024, in the United States, 5-6 new products in each quarter were planned to be launched by Alembic Pharmaceuticals, a Vadodara-based generics drugmaker.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/