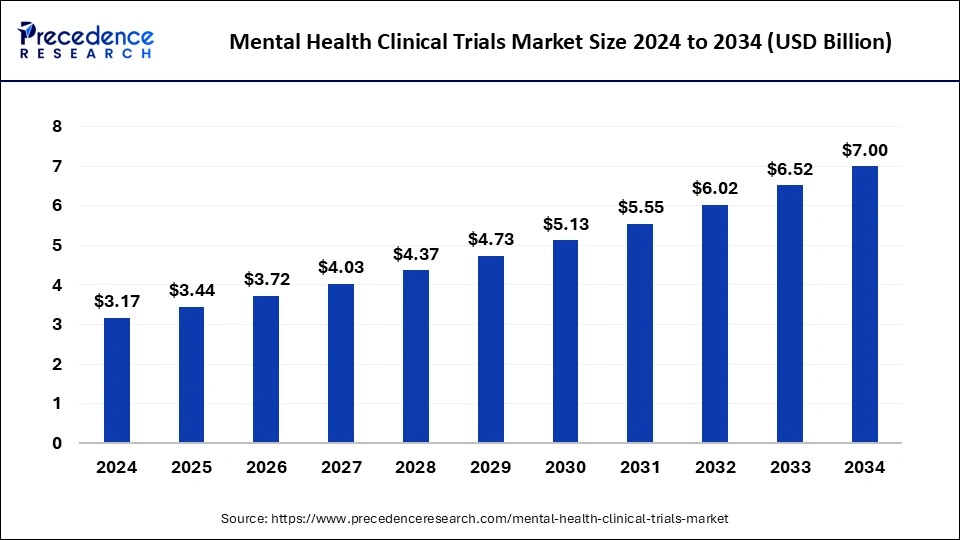
The global mental health clinical trials market size reached USD 2.93 billion in 2023 and is expected to surpass around USD 6.52 billion by 2033, expanding at a CAGR of 8.32% from 2024 to 2033.
Mental Health Clinical Trials Market Key Points
- North America dominated the mental health clinical trials market with the largest revenue share of 51% in 2023.
- Asia Pacific is expected to grow at the highest CAGR of 8.82% during the forecast period.
- By phase, the phase III segment has contributed more than 33% of revenue share in 2023.
- By phase, the phase I segment is projected to grow at the solid CAGR of 8.71% during the forecast period.
- By study design, the interventional segment has captured more than 66% of revenue share in 2023.
- By study design, the observational segment is expected to grow at the highest CAGR in the market during the forecast period.
- By sponsor, the pharmaceutical & biopharmaceutical companies segment has recorded the biggest revenue share of 40% in 2023.
- By sponsor, the government agencies segment is growing at a highest CAGR of 8.53% during the forecast period.
- By disorder, the anxiety disorders segment dominated the market in 2023.
- By disorder, the depression segment is expected to grow at the highest CAGR in the market during the forecast period.
The Mental Health Clinical Trials Market encompasses a vital aspect of healthcare research, focusing on evaluating treatments and interventions for various mental health disorders such as depression, anxiety, schizophrenia, and bipolar disorder. These trials are critical for advancing psychiatric care by testing the efficacy and safety of new medications, therapies, and behavioral interventions. Key players in this market include pharmaceutical companies, academic research institutions, contract research organizations (CROs), and regulatory bodies collaborating to conduct rigorous clinical trials.
Get a Sample: https://www.precedenceresearch.com/sample/4587
Several growth factors drive the Mental Health Clinical Trials Market. Firstly, there’s a notable increase in the prevalence of mental health disorders globally, particularly depression and anxiety, among both younger and older demographics. This rising prevalence underscores the urgent need for new and more effective treatment options, thereby driving the demand for clinical trials. Advances in neurobiology and psychopharmacology have also been significant growth drivers. These advancements have led to the development of novel drug targets and formulations, stimulating research into new therapeutic approaches.
Regionally, the market exhibits distinct insights influenced by healthcare infrastructure, regulatory frameworks, and the prevalence of mental disorders. In North America, the market dominance is attributed to robust healthcare systems, high investments in research and development, and supportive regulatory environments such as FDA approvals. Europe follows closely, with strong initiatives in psychiatric research supported by well-established healthcare systems in countries like the UK, Germany, and France. Meanwhile, Asia-Pacific is emerging as a key region due to increasing healthcare expenditure, rising awareness, and a growing patient population.
Mental Health Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 6.52 Billion |
| Market Size in 2023 | USD 2.93 Billion |
| Market Size in 2024 | USD 3.17 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 8.32% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Sponsor, Disorder, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Mental Health Clinical Trials Market Dynamics
Drivers of growth in this market include the demand for personalized medicine, which emphasizes tailored treatment approaches based on individual patient characteristics. Technological advancements such as digital health technologies and telemedicine platforms are also driving growth by enhancing trial efficiency, patient recruitment, and data collection. Moreover, increasing patient advocacy and awareness campaigns are reducing stigma associated with mental health disorders, encouraging more individuals to participate in clinical trials. Strategic collaborations between pharmaceutical companies, research institutions, and patient advocacy groups are fostering innovation and accelerating trial timelines.
Opportunities abound in expanding therapeutic areas beyond traditional disorders like depression and anxiety. There’s potential in exploring emerging disorders such as PTSD and OCD, as well as leveraging digital therapeutics and mobile health apps to enhance trial efficiency and patient engagement. Emerging markets offer opportunities for cost-effective trials and access to diverse patient populations. Advancements in genomics and biomarker research also present opportunities to develop personalized treatments and stratify patient populations based on genetic profiles.
Despite these opportunities, the Mental Health Clinical Trials Market faces several challenges. Patient recruitment and retention remain daunting due to stigma, patient reluctance, and stringent eligibility criteria. Regulatory complexities across different regions and ethical considerations in mental health research can delay trial initiation and increase operational costs. Moreover, high placebo response rates in mental health trials and subjective efficacy endpoints pose challenges in demonstrating treatment efficacy and obtaining regulatory approval. Ensuring data integrity, managing complex datasets, and conducting robust statistical analysis are additional challenges that impact trial outcomes and regulatory acceptance.
Read Also: Blockchain AI Market Size to Surpass USD 3,718.34 Mn by 2033
Mental Health Clinical Trials Market Companies
- ICON Plc
- Eli Lilly Company
- Caidya
- Syneous Health
- Novo Nordisk
- Pharmaceutical Product Development, LLC
- Parexel International Corporation
- Corcept
- Labcorp Drug Development
- IQVIA
Recent Developments
- In April 2024, the international pharmaceutical business Otsuka Pharmaceutical Development & Commercialization, Inc. (Otsuka) announced the opening of My Mental Health Journey, a longitudinal registry project with the goal of advancing mental health and depression research.
- In February 2024, the Bazouki Group declared the beginning of a new collaboration on a clinical study of a therapeutic ketogenic diet for bipolar illness with McLean Hospital in Belmont, Massachusetts. Investigating nutritional ketosis as a unique therapeutic approach and a crucial tool for comprehending the underlying processes of bipolar disease.
Segment Covered in the Report
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Others
By Sponsor
- Pharmaceutical & Biopharmaceutical Companies
- Government Agencies
- Others
By Disorder
- Anxiety Disorders
- Depression
- Bipolar Affective Disorder
- Dissociation & Dissociative Disorders
- Schizophrenia
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
