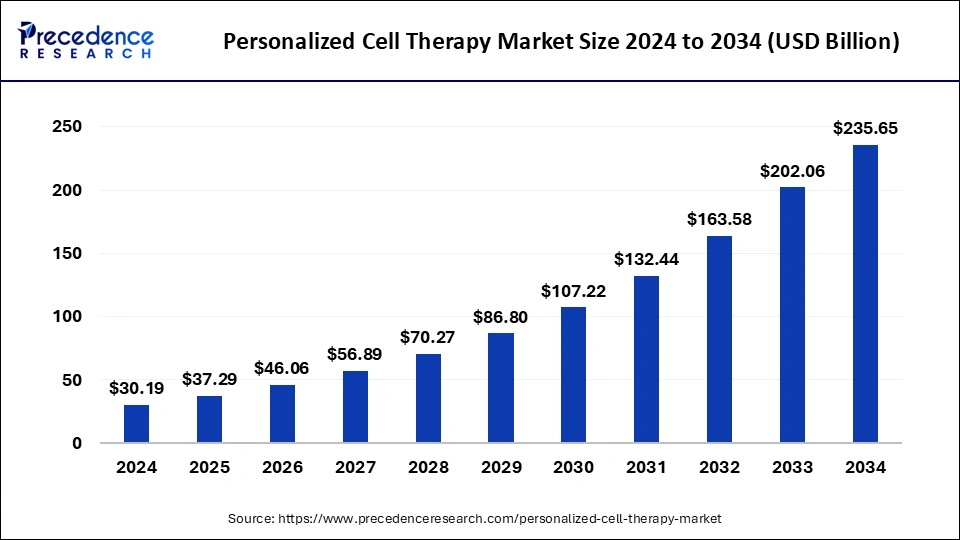
The global personalized cell therapy market size reached USD 24.44 billion in 2023 and is projected to surpass around USD 202.06 billion by 2033, at a solid CAGR of 23.52% from 2024 to 2033.
Key Points
- The North America personalized cell therapy market size reached USD 11.98 billion in 2023 and is expected to attain around USD 100.02 billion by 2033, poised to grow at a CAGR of 23.64% between 2024 and 2033.
- North America dominated the personalized cell therapy market in 2023.
- Asia Pacific is expected to host the fastest-growing market during the projected period.
- By technique, the platelet transfusions segment dominated the market in 2023.
- By technique, the bone marrow transplantation segment is expected to grow at the highest CAGR in the market during the forecast period.
- By therapeutic area, the cardiovascular diseases segment led the market in 2023.
- By therapeutic area, the neurological disorders segment is expected to grow at a significant rate during the forecast period.
The Personalized Cell Therapy Market involves tailoring treatments to individual patients based on their genetic profile and medical history. This approach aims to enhance treatment efficacy while minimizing adverse effects compared to conventional therapies. The market has gained traction due to advancements in biotechnology, the rise in chronic diseases, and the growing trend toward precision medicine.
Get a Sample: https://www.precedenceresearch.com/sample/4579
Growth Factors
Several factors drive the growth of personalized cell therapy. Technological advancements in gene editing, cell culture techniques, and biomaterials have revolutionized treatment development. Rising incidences of chronic diseases like cancer and autoimmune disorders also bolster demand for targeted therapies. Additionally, the shift towards precision medicine, which focuses on customized treatments based on genetic and molecular characteristics, propels market expansion. Supportive regulatory frameworks and substantial investments in research and development further accelerate market growth.
Region Insights
Geographically, the market spans North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America leads due to advanced research infrastructure, favorable reimbursement policies, and early adoption of innovative therapies. Europe follows with a supportive regulatory environment and increasing collaborations between research institutions and biopharmaceutical companies. Asia Pacific is emerging rapidly, driven by improved healthcare infrastructure and rising awareness of personalized treatments. Latin America and MEA present growing opportunities with increasing healthcare investments and adoption of advanced medical technologies.
Personalized Cell Therapy Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 202.06 Billion |
| Market Size in 2023 | USD 24.44 Billion |
| Market Size in 2024 | USD 30.19 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 23.52% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Technique, Therapeutic Area, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Personalized Cell Therapy Market Dynamics
Drivers of Growth
Key drivers include rising patient demand for effective therapies with minimal side effects and continuous advancements in cell biology and genomics. Collaborations among pharmaceutical companies, research institutions, and healthcare providers foster innovation and accelerate market expansion. A shift towards patient-centric healthcare models also boosts demand for personalized cell therapies tailored to individual patient needs.
Opportunities
The market offers significant growth opportunities, particularly in addressing unmet medical needs in rare diseases and developing next-generation therapies. Expanding into emerging markets with increasing healthcare expenditure and growing patient awareness of personalized medicine also presents opportunities. Innovations in cell types, engineering techniques, and delivery methods further enhance therapeutic outcomes and expand market reach.
Challenges
Despite promising growth prospects, the market faces challenges. High development and manufacturing costs of personalized cell therapies pose affordability issues for patients. Navigating complex regulatory landscapes across different regions can delay market entry and increase development costs. Ensuring scalability, reproducibility, and long-term safety of personalized therapies remains a scientific and technical challenge. Ethical considerations related to genetic manipulation, informed consent, and patient privacy also impact market acceptance and adoption.
Read Also: Nasal Drug Delivery Technology Market Size, Growth, Report by 2033
Personalized Cell Therapy Market Companies
- AbbVie Inc.
- Bausch Health Companies Inc.
- Teva Pharmaceutical Industries Ltd.
- Cipla Inc.
- Lupin
- Sun Pharmaceuticals Industries Ltd.
- Hikma Pharmaceuticals PLC
- AstraZeneca
- GSK Plc.
- Pfizer Inc.
- Amneal Pharmaceuticals LLC.
- Alvogen
- F. Hoffmann-La Roche Ltd
- Amgen Inc.
- Jazz Pharmaceuticals, Inc.
- Amicus Therapeutics, Inc
- MeiraGTx Limited
- Rocket Pharmaceuticals, Inc.
- Gilead Sciences, Inc.
Recent Developments
- In October 2023, Aurion Biotech declared that the first patient in its phase 1/2 trial of AURN001, an experimental cell therapy intended to treat corneal edema resulting from corneal endothelial dysfunction, has received a dose. A press statement from the firm states that AURN001 is a blend of Y-27632, a Rho kinase inhibitor, and neltependocel, which are allogenic human corneal endothelial cells.
- In June 2023, Vertex Pharmaceuticals Incorporated and Lonza announced a strategic partnership aimed at facilitating the production of Vertex’s range of fully differentiated insulin-producing islet cell therapies for individuals with type 1 diabetes. The current focus of this collaboration is on the VX-264 and VX-880 programs, which are presently undergoing clinical trials.
- In May 2023, Janssen Biotech, Inc., one of the Janssen Pharmaceutical Companies of Johnson & Johnson, announced that it has entered into a worldwide collaboration and license agreement with Cellular Biomedicine Group Inc. (CBMG) to develop, manufacture, and commercialize next-generation chimeric antigen receptor (CAR) T-cell therapies for the treatment of B-cell malignancies.
- In March 2023, Adaptimmune Therapeutics plc and TCR² Therapeutics Inc. announced entry into a definitive agreement under which Adaptimmune will combine with TCR² in an all-stock transaction to create a preeminent cell therapy company focused on treating solid tumors. The combination provides extensive benefits for clinical development and product delivery supported by complementary technology platforms.
Segment Covered in the Report
By Technique
- Platelet Transfusions
- Bone Marrow Transplantation
- Packed Red Cell Transfusions
- Organ Transplantation
By Therapeutic Area
- Cardiovascular Diseases
- Neurological Disorders
- Inflammatory Diseases
- Diabetes
- Cancer
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
