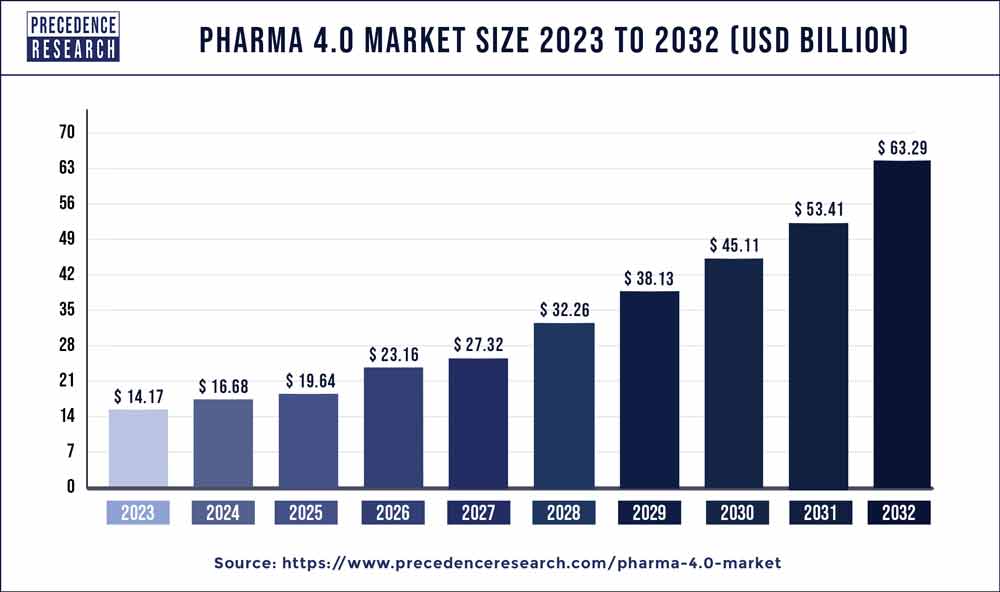
The global pharma 4.0 market size is calculated at USD 16.68 billion in 2023 and is projected to hit around USD 63.29 billion by 2032 with a noteworthy CAGR of 18.1% from 2024 to 2032.
The Pharma 4.0 market is driven by the growing digital transformation, the emergence of advanced technologies such as AI, IoT and big data analytics and rising technological advancements. Additionally, the growing investment in the pharmaceutical industry is expected to propel the market expansion during the forecast period.
Pharma 4.0 refers to the application of the fourth industrial revolution (industry 4.0) technologies and principles in the pharmaceutical industry. It represents the integration of advanced digital technologies to enhance and optimize various aspects of pharmaceutical manufacturing, distribution, and healthcare. The goal of Pharma 4.0 is to create a more interconnected, data-driven, and flexible pharmaceutical ecosystem. This approach can lead to increased efficiency, reduced costs, improved quality control and faster development and delivery of pharmaceutical products.
Additionally, Pharma 4.0 has the potential to enhance regulatory compliance, traceability, and overall patient safety within the pharmaceutical industry. The growing partnership in the industry is expected to propel market revenue growth during the study timeframe. For instance, in July 2022, with the help of Red Hat’s industry-leading enterprise platforms and application services, which are based on Red Hat Enterprise Linux, ABB and Red Hat announced a worldwide cooperation that would allow industries utilizing ABB’s process automation and industrial software to grow quickly and adaptably.
Download the Sample Report ( Including Full TOC, List of Table & Figures, Chart): https://www.precedenceresearch.com/sample/3187
Pharma 4.0 Market Statistics
- North America dominated the market with the highest market share of 35.46% in 2023.
- Asia Pacific is expected to witness significant growth in the market during the predicted timeframe.
- By technology, the Internet of Things (IoT) segment captured the biggest revenue share of 44.70% in 2023.
- By application, manufacturing has held the largest market share of 54.61% in 2023.
- By end-user, the pharmaceutical companies segment dominated the market in 2023.
Regional Stance
Pharma 4.0 Market Share, By Region 2023 %
| Region | Market Share (%) |
| North America | 35.46% |
| Europe | 26.16% |
| Asia-Pacific | 23.45% |
| Latin America | 8.95% |
| Middle East and Africa | 6.09% |
North America is expected to dominate the market during the forecast period. The growth in the North American region is attributed to the presence of major pharmaceutical companies including Pfizer, Sanofi, Johnson & Johnson, AbbVie, AstraZeneca and others. These companies are involved in the production of various disease medicines. To efficiently manage the production of the medicine and its value chain, these companies utilize advanced automation software which is expected to drive the industry penetration.
- The U.S. pharma 4.0 market size was valued at USD 3.8 billion in 2023 and is anticipated to reach around USD 15.92 billion by 2032, poised to grow at a CAGR of 17.3% from 2024 to 2032.
- For instance, in December 2023, BigHat Biosciences and AbbVie Inc. established a research partnership to find and create next-generation therapeutic antibodies in the fields of neurological and cancer. BigHat will work closely with AbbVie to assist in the creation and selection of high-quality antibodies for a variety of therapeutic targets. It will do this by utilizing its Milliner TM platform, a suite of machine learning technologies coupled with a high-speed wet lab. This partnership is yet further evidence of their dedication to incorporating AI/ML-based methods into drug research and development, as they work to quicken their pipeline for cancer and neurology and produce better medications more quickly.
Asia Pacific is expected to grow at the highest CAGR during the forecast period. The regional growth is attributed to the growing healthcare expenditure. The increasing healthcare expenditure in countries like China and India has led to a growing emphasis on the quality and efficiency of healthcare delivery. Pharma 4.0 technologies play a crucial role in improving pharmaceutical manufacturing processes and healthcare services. Moreover, the governments in the region have been actively promoting digital transformation and industry 4.0 initiatives, including in the pharmaceutical sector. Supportive policies and initiatives can incentivize pharmaceutical companies to invest in advanced technologies. Thus, this is expected to propel the market expansion in the Asia Pacific region.
Scope of Pharma 4.0 Market
| Report Coverage | Details |
| Market Size in 2023 | USD 14.17 Billion |
| Market Size by 2032 | USD 63.29 Billion |
| Growth Rate from 2024 to 2032 | CAGR of 18.1% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2023 To 2032 |
| Segments Covered | By Technology, By Application, and By End-User |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Pharma 4.0 Market Report Highlights:
Technology Insights
The cloud computing segment is expected to dominate the market during the forecast period. Cloud computing provides scalable and secure data storage solutions. In Pharma 4.0, where large volumes of data are generated from various sources, including IoT devices and manufacturing processes, cloud storage facilitates efficient data management and accessibility. In addition, these platforms enable real-time collaboration among stakeholders, including pharmaceutical companies, suppliers, and regulatory bodies. This collaborative environment supports the seamless sharing of data, research findings, and insights, fostering innovation and efficiency.
Application Insights
The drug discovery and development segment is expected to capture a significant market share over the forecast period. Pharma 4.0 leverages advanced analytics, artificial intelligence and machine learning to analyze vast datasets in real time. In drug discovery, this means more efficient analysis of biological data, identification of potential drug targets, and prediction of compound activities, thereby accelerating the drug discovery process. In addition, Pharma 4.0 enables the implementation of virtual drug development processes. This includes virtual clinical trials, where technologies like telemedicine, wearables and remote monitoring are utilized to gather data from participants in a more decentralized and patient-friendly manner. Thereby, driving the market growth.
Global Pharma 4.0 Market, By Application, 2020-2023 (USD Million)
| Application | 2020 | 2021 | 2022 | 2023 |
| Drug Discovery and Development | 2,450.49 | 2,885.68 | 3,401.05 | 4,015.29 |
| Clinical Trials | 1,487.80 | 1,746.78 | 2,052.60 | 2,416.06 |
| Manufacturing | 4,813.46 | 5,632.47 | 6,596.35 | 7,738.24 |
End User Insights
The pharmaceutical companies segment is expected to hold the dominating position in the market over the forecast period. Pharma 4.0 emphasizes data-driven decision-making processes. Pharmaceutical companies leverage advanced analytics to analyze large datasets generated during drug development, manufacturing and distribution. This data-driven approach enhances decision accuracy and efficiency. Additionally, cloud computing, IoT, and blockchain technologies enhance visibility and traceability in the pharmaceutical supply chain. The company can track the movement of materials and products in real-time, reducing the risk of counterfeit drugs and improving overall supply chain efficiency.
Pharma 4.0 Market Dynamics:
Driver
Improved supply chain management
Medicine with the use of Pharma 4.0 technologies, supply chain monitoring can be done in real-time, improving distribution and inventory tracking and management. A medication that uses RFID or NFC technology integrated into the primary packaging can be tracked step-by-step from the manufacturing facility to its destination. Each step is recorded in an unchangeable manner in the blockchain, making it impossible for a counterfeit to exist—even if the secondary packaging is replicated. This is an example of an improved supply chain or blockchain. Therefore, one of the key drivers of the Pharma 4.0 market throughout the projected time will be enhanced supply chain management.
Restraint
High cost and intellectual property protection
Software, hardware, and human resources must all be heavily invested in before Pharma 4.0 technologies can be implemented. For smaller pharmaceutical businesses or those with fewer resources, cost is a major barrier. In addition, concerns about intellectual property protection are developing as digital technologies are used more and more in the pharmaceutical industry. Making sure that intellectual property is sufficiently safeguarded is a big task that needs constant care. Applications for patents are available for AI system algorithms and the hardware parts that make them work. Where the output of such a system might be covered by copyright law.
Opportunities
Growing Collaboration
The increasing collaboration is expected to offer a lucrative opportunity for market revenue development over the forecast period. For instance, in March 2022, a partnership agreement was initiated by Siemens Ltd., China and Triastek, Inc. to supply digital technology to the pharmaceutical business worldwide. It is anticipated that combining Siemens’ automation and digitization experience with Triastek’s 3D printing and digital pharmaceutical technology would result in novel and revolutionary approaches to the development and production of medications. Through this partnership, Triastek and Siemens will make use of each other’s technical advantages, competencies, and skills to meet the demands of the pharmaceutical business and enhance patient outcomes. While combined with Siemens’ cutting-edge automation and digital technologies, Triastek’s unique pharmaceutical MED (Melt Extrusion Deposition) 3D printing platform technology for mass production and research and development will speed up the development of new products and make continuous manufacturing approaches, paperless production, real-time product release, and equipment and facility maintenance easier. It is anticipated that these advances would increase manufacturing line throughput while preserving the highest standards of product quality. Furthermore, Triastek intends to create an intelligent pharmaceutical production center in partnership with Siemens, where digital twins, smart factories, and virtual laboratory technologies will be integrated into every facet of pharmaceutical operations.
Read Also: PCR Plastic Packaging Market Size To Worth USD 47.48 Bn By 2033
Pharma 4.0 Market Companies
- Microsoft Corporation
- Oracle Corporation
- ABB
- Honeywell International Inc.
- Cisco Systems, Inc.
- Siemens Healthcare GmbH
- GE Healthcare
- IBM Corporation
- Amazon Web Services, Inc.
Recent Developments
- In July 2023, Lupin’s fully owned subsidiary in Germany Hormosan Pharma GmbH announced the launch of Luforbec 100/6 (beclometasone 100 µg / formoterol 6 µg), it is a metered dose inhaler with the fixed combination for the treatment of chronic obstructive pulmonary disease (COPD) and adult asthma in Germany.
- In July 2023, privately owned Angelini Industries Angelini Pharma announced the launch of an initiative designed “LIFE-GREENAPI,” manufactured to pharma production practices with low environmental impact.
- In June 2023, a leading provider of lifecycle services and system validation “Valspec” was a key partner in Genentech’s Clinical Supply Center (CSC) project, which is recently awarded the Facility of the Year 2023 Pharma 4.0™ Category Winner Award by ISPE®. Valspec innovated the testing matrix based on user needs and collaboration with the Automation, Equipment C&Q, System Owners, and QA project teams to take approval.
