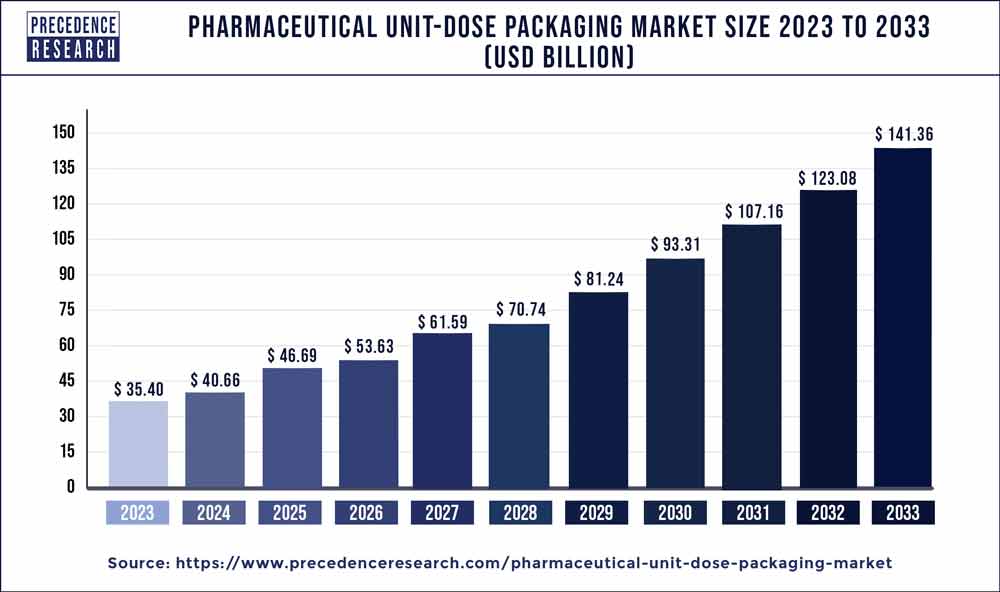
The global pharmaceutical unit-dose packaging market size was valued at USD 35.40 billion in 2023 and is projected to surpass around USD 141.36 billion by 2033 with a CAGR of 14.85% from 2024 to 2033.
Key Points
- North America held the largest market share of 34% in 2023.
- Asia Pacific is observed to witness the fastest rate of expansion during the forecast period.
- By material, the plastic segment dominated the market with the highest revenue share of 48% in 2023.
- By material, the glass material segment holds a significant share of the market.
- By product type, the vials segment has captured the largest market share of 30% in 2023.
- By product type, the blisters segment is observed to witness the fastest rate of expansion during the forecast period.
- By end-use, the oral segment dominated the market in 2023.
The Pharmaceutical Unit-dose Packaging Market refers to the segment of the pharmaceutical packaging industry focused on the production and distribution of medications in single-dose packaging formats. These unit-dose packaging solutions are designed to contain a single dose of medication, providing convenience, accuracy, and safety for patients and healthcare providers. The market encompasses various packaging formats such as blister packs, pouches, vials, ampoules, and sachets, each tailored to specific medication types and administration routes.
In recent years, the demand for pharmaceutical unit-dose packaging has surged due to several factors, including the growing emphasis on patient safety, regulatory requirements for medication adherence, and the rise of personalized medicine. Additionally, the aging population, increasing prevalence of chronic diseases, and advancements in pharmaceutical formulations have further propelled the adoption of unit-dose packaging solutions.
Get a Sample: https://www.precedenceresearch.com/sample/3896
Growth Factors
Several key factors are driving the growth of the pharmaceutical unit-dose packaging market. Firstly, stringent regulatory standards and guidelines imposed by regulatory authorities such as the FDA and EMA regarding medication safety and compliance have spurred pharmaceutical companies to adopt unit-dose packaging solutions. These regulations mandate the use of tamper-evident, child-resistant, and patient-friendly packaging, driving the demand for unit-dose formats.
Secondly, the rising demand for convenience and portability in medication administration has fueled the adoption of unit-dose packaging. Patients prefer pre-packaged, single-dose formats for ease of use, especially in scenarios where precise dosing and medication adherence are crucial, such as in elderly care facilities and home healthcare settings.
Moreover, the increasing prevalence of chronic diseases, coupled with the need for personalized medicine, has driven pharmaceutical companies to invest in unit-dose packaging technologies. These technologies enable the precise packaging of individualized doses, catering to the specific needs of patients and enhancing medication efficacy.
Furthermore, technological advancements in packaging materials and manufacturing processes have expanded the capabilities of unit-dose packaging, allowing for enhanced barrier properties, extended shelf life, and compatibility with a wide range of drug formulations. Innovations such as smart packaging with RFID tags and temperature-sensitive indicators are also contributing to market growth by ensuring product integrity and traceability throughout the supply chain.
Region Snapshot
The pharmaceutical unit-dose packaging market exhibits a global presence, with significant growth opportunities across various regions. North America currently dominates the market, driven by stringent regulatory standards, high healthcare expenditure, and the presence of major pharmaceutical companies. The region is characterized by a strong focus on medication safety and adherence, driving the adoption of unit-dose packaging solutions.
Europe follows closely behind, propelled by similar regulatory dynamics and a growing aging population. Countries like Germany, France, and the UK are key contributors to market growth, supported by robust healthcare infrastructure and increasing investments in pharmaceutical R&D.
Asia Pacific represents a lucrative market for pharmaceutical unit-dose packaging, fueled by rapid urbanization, expanding access to healthcare services, and increasing disposable income levels. Countries such as China, India, and Japan are witnessing significant growth in pharmaceutical manufacturing and consumption, driving demand for unit-dose packaging solutions to ensure medication safety and compliance.
Latin America and the Middle East & Africa also present growth opportunities, albeit at a slower pace, due to improving healthcare infrastructure, rising healthcare expenditure, and government initiatives to enhance medication access and affordability.
Pharmaceutical Unit-dose Packaging Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 14.85% |
| Global Market Size in 2023 | USD 35.40 Billion |
| Global Market Size by 2033 | USD 141.36 Billion |
| U.S. Market Size in 2023 | USD 8.43 Billion |
| U.S. Market Size by 2033 | USD 33.64 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Material, By Product, and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
SWOT Analysis:
- Strengths:
- Enhanced Medication Safety: Unit-dose packaging ensures accurate dosing, reduces the risk of medication errors, and enhances patient safety.
- Compliance with Regulatory Standards: Unit-dose packaging solutions align with stringent regulatory requirements for tamper-evidence, child-resistance, and patient-friendly packaging.
- Customization and Personalization: Unit-dose packaging technologies enable the customization of medication doses, catering to individual patient needs and preferences.
- Weaknesses:
- Higher Production Costs: Unit-dose packaging may entail higher production costs compared to bulk packaging formats, potentially impacting profit margins for pharmaceutical companies.
- Limited Compatibility: Certain drug formulations may not be suitable for unit-dose packaging, limiting the applicability of these solutions across all medication types.
- Environmental Concerns: The use of single-dose packaging materials may raise environmental concerns related to waste generation and sustainability.
- Opportunities:
- Emerging Markets: Expansion opportunities exist in emerging markets like Asia Pacific and Latin America, driven by increasing healthcare infrastructure development and rising pharmaceutical consumption.
- Technological Innovations: Continued advancements in packaging materials and technologies offer opportunities to enhance product performance, extend shelf life, and improve supply chain efficiency.
- Collaborative Partnerships: Collaboration between pharmaceutical companies, packaging manufacturers, and regulatory agencies can drive innovation and address market needs more effectively.
- Threats:
- Regulatory Challenges: Evolving regulatory landscapes and compliance requirements pose challenges for pharmaceutical companies in adopting and implementing unit-dose packaging solutions.
- Competitive Pressure: Intense competition among pharmaceutical companies and packaging manufacturers may exert downward pressure on pricing and profit margins.
- Supply Chain Disruptions: Disruptions in the global supply chain, such as raw material shortages or logistics issues, can impact the availability and cost of unit-dose packaging materials.
Read Also: IT Devices Market Size to Hit Around USD 4,436.53 Bn by 2033
Recent Developments
- In December 2023, Sun Pharmaceutical Industries Ltd launched the ‘es-omeprazole’ the chiral version of the anti-ulcerant drug omeprazole. This version of the medicine is lesser in side effects and is considered more efficient than the molecule itself.
- In 2023, Gerresheimer Gx Solutions is designing packaging solutions for complex injectable drugs. Gerresheimer Gx Solutions working on the primary packaging solution for sensitive injectable drugs. The organization integrated expertise in the specialized team known as Gx Solutions.
- In January 2024, a clean technology company “Loop Industries”, launched the latest pharmaceutical packaging bottles in partnership with Bormioli Pharma. The bottle is made up of 100% recycled virgin quality Loop polyethylene terephthalate (PET) resin.
Pharmaceutical Unit-dose Packaging Market Companies
- Pfizer Inc
- Bristol-Myers Squibb Company
- Merck & Co. Inc.
- AbbVie Inc.
- Gerresheimer AG
- Comar LLC
- Amcor Plc.
- Johnson & Johnson
- UDG Healthcare Plc
- Berry Global, Inc.
Segments Covered in the Report
By Material
- Plastics
- Glass
- Paper and Paperboard
- Metal
By Product
- Vials
- Syringe and cartridge
- Ampoules
- Blisters
By End-use
- Ophthalmic
- Injectable
- Biologics
- Wound Care
- Respiratory Therapy
- Orals
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/

