The global recombinant antibodies market size surpassed USD 12.40 billion in 2023 and is expected to rake around USD 23.34 billion by 2033 growing at a CAGR of 6.53% from 2024 to 2033.
Key Points
- North America dominated the market with the largest share in 2023.
- By type, the single-chain antibody segment dominated the market in 2023.
- By application, the pharmaceutical & biotechnology segment held the largest share of the market in 2023 and the segment is expected to sustain the position throughout the forecast period.
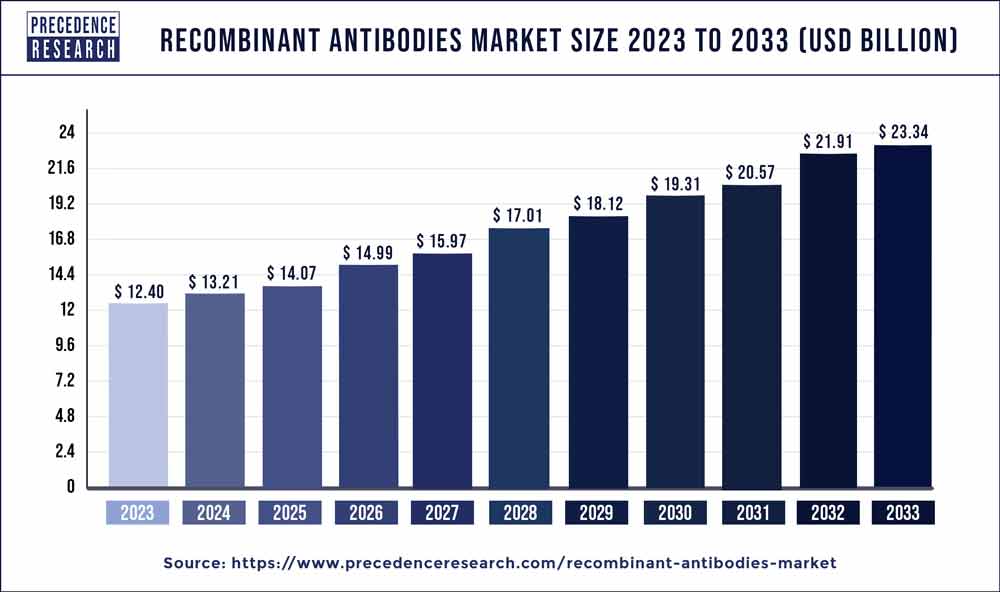
The recombinant antibodies market has experienced significant growth in recent years, driven by advancements in biotechnology and the increasing demand for targeted therapeutics in various medical applications. Recombinant antibodies, also known as engineered antibodies, are antibodies produced through genetic engineering techniques, offering advantages such as improved specificity, reduced immunogenicity, and enhanced therapeutic efficacy compared to traditional antibodies. These antibodies are widely used in areas such as cancer therapy, autoimmune diseases, infectious diseases, and diagnostic assays. The market encompasses a diverse range of players including biopharmaceutical companies, academic research institutions, contract research organizations, and diagnostic companies, contributing to the expansion and innovation within the sector.
Get a Sample: https://www.precedenceresearch.com/sample/3889
Growth Factors
Several key factors contribute to the growth of the recombinant antibodies market. Firstly, the rising prevalence of chronic diseases, such as cancer and autoimmune disorders, has fueled the demand for targeted therapies, where recombinant antibodies play a crucial role. Additionally, technological advancements in recombinant DNA technology, protein engineering, and antibody screening methodologies have facilitated the development of novel recombinant antibody products with enhanced therapeutic properties and improved safety profiles. Moreover, increasing investments in research and development activities by pharmaceutical and biotechnology companies to expand their antibody-based therapeutic pipelines further stimulate market growth. Furthermore, the growing adoption of personalized medicine approaches and the increasing focus on precision medicine have led to a surge in demand for recombinant antibodies tailored to individual patient profiles, driving market expansion.
Region Snapshot
The recombinant antibodies market exhibits a global presence, with significant growth opportunities across various regions. North America dominates the market, primarily attributed to the presence of a well-established biopharmaceutical industry, strong research infrastructure, and favorable regulatory frameworks supporting the development and commercialization of recombinant antibody-based therapeutics. The region is characterized by a high level of investment in research and development activities and a robust healthcare system, contributing to the rapid adoption of advanced biologics, including recombinant antibodies. Europe follows closely behind, driven by increasing healthcare expenditure, growing emphasis on personalized medicine, and expanding biotechnology sector. Additionally, the Asia-Pacific region is poised for substantial growth, propelled by improving healthcare infrastructure, rising disposable incomes, and expanding pharmaceutical markets in countries such as China, India, and Japan. Emerging economies in Latin America and the Middle East & Africa also present untapped opportunities for market players, supported by growing investments in healthcare infrastructure and rising awareness about advanced therapeutics.
Recombinant Antibodies Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 6.53% |
| Global Market Size in 2023 | USD 12.40 Billion |
| Global Market Size by 2033 | USD 23.34 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Type and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
SWOT Analysis:
- Strengths:
- High specificity and efficacy: Recombinant antibodies offer superior target specificity and therapeutic efficacy, making them effective in treating various diseases with minimal off-target effects.
- Technological advancements: Continuous innovation in recombinant DNA technology and antibody engineering techniques enhances the development of novel antibody products with improved properties and functionalities.
- Diverse applications: Recombinant antibodies find applications across a broad spectrum of therapeutic areas and diagnostic assays, ranging from cancer therapy to infectious disease diagnostics, providing versatility and market opportunities.
- Established market players: The market benefits from the presence of established biopharmaceutical companies and research institutions with extensive experience in antibody development, fostering expertise and collaborations within the industry.
- Weaknesses:
- High development costs: The research and development process for recombinant antibodies involves substantial investment in technology, resources, and clinical trials, leading to high development costs and prolonged timelines for product commercialization.
- Regulatory challenges: The stringent regulatory requirements governing the development and approval of biologics, including recombinant antibodies, pose challenges for market entry and commercialization, particularly for smaller companies and startups.
- Immunogenicity concerns: Despite advancements, immunogenicity remains a potential concern with recombinant antibodies, as immune responses against the therapeutic agent may impact efficacy and safety, necessitating rigorous preclinical and clinical evaluation.
- Opportunities:
- Expansion in emerging markets: The growing healthcare infrastructure and increasing demand for advanced therapeutics in emerging economies present lucrative opportunities for market expansion and penetration.
- Personalized medicine: The rising trend towards personalized medicine and precision therapeutics creates a demand for customized recombinant antibody-based treatments tailored to individual patient profiles, driving innovation and market growth.
- Collaborative partnerships: Collaborations between biopharmaceutical companies, academic institutions, and contract research organizations facilitate the development of novel recombinant antibody products and accelerate the translation of research findings into clinical applications.
- Threats:
- Competition from alternative therapies: The recombinant antibodies market faces competition from alternative therapeutic modalities such as small molecules, cell-based therapies, and gene therapies, posing a threat to market share and adoption.
- Intellectual property challenges: Patent expirations, patent disputes, and intellectual property infringement issues may affect market exclusivity and competitive advantage, leading to increased competition and pricing pressures.
- Regulatory uncertainties: Evolving regulatory landscapes and changing approval requirements for biologics and biosimilars introduce uncertainties and compliance challenges for market players, impacting product development and commercialization strategies.
Read Also: Automotive Embedded Telematics Market Size, Growth, Report 2033
Recent Developments
- In November 2023, the novel GS Effex® cell line from Lonza was introduced to facilitate the production of therapeutic antibodies with increased potency. The GS Effex® cell line was created in response to urgent market demands brought on by the move toward more advanced therapeutic antibodies. GS Effex®, which is derived from Lonza’s top-performing GS Xceed® cell line, integrates well with Lonza platforms. When coupled with robust cell growth and the capacity to generate potent therapeutic antibodies, this unique cell line offers a solution for therapeutic development spanning from early-stage research to commercial manufacture. The Fc domains produced by this scalable, stable, and prolific cell line are fucose-free, increasing the therapeutic efficacy and function of the final antibody that is used in antibody-dependent cellular cytotoxicity (ADCC).
- In April 2023, the mAbModsTM chimeric anti-mouse PD1 and PD-L1 recombinant antibodies are designed to target mouse-programmed cell death receptor 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) in vivo functional studies. Leinco Technologies is a biotechnology company that specializes in producing antibodies, recombinant proteins, ELISA kits, and second-step reagents. These are the first of a planned series of mouse anti-mouse antibodies that will be introduced in the upcoming months. They are intended to reduce the immunogenicity issues that arise when employing antibodies from non-mouse species in mouse-based investigations, especially when the usage of the antibodies is protracted. These species include rats and hamsters.
- In August 2023, a new COVID-19 monoclonal antibody therapy for the prevention of SARS-CoV-2 infection is being supported in its clinical development, clinical manufacturing, and regulatory licensure process by Regeneron Pharmaceuticals, Inc. and the Biomedical Advanced Research and Development Authority (BARDA). The deal is a component of the U.S. Department of Health and Human Services (HHS) “Project NextGen,” which aims to develop a pipeline of novel COVID-19 vaccines and treatments.
Recombinant Antibodies Market Companies
- Abnova
- BBI Solutions
- F. Hoffmann-La Roche
- BIOLEGEND
- BD Biosciences
- Creative Biolabs
- Merck KGaA
- Bio-Rad Laboratories
- Abcam
- SinoBiological
- GE Healthcare
- ProMab Biotechnologies
- Beckman Coulter
Segments Covered in the Report
By Type
- Chimeric Antibody
- Full Human Antibody
- Humanized Antibody
- Single Chain Antibody
- Bispecific Antibody
By Application
- Pharmaceutical & Biotechnology
- Hospitals & Diagnostic Laboratories
- Research Institutes
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
