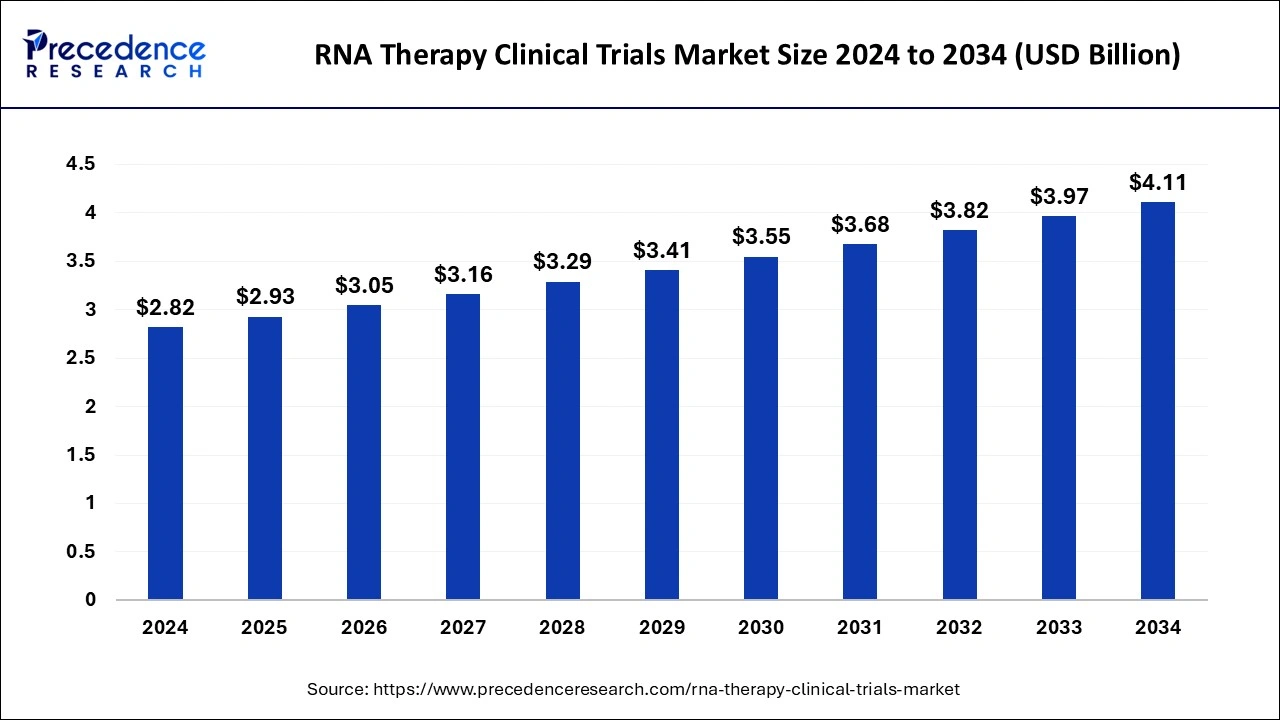
The global RNA therapy clinical trials market size reached USD 2.72 billion in 2023 and is predicted to surpass around USD 3.97 billion by 2033, expanding at a CAGR of 3.86% from 2024 to 2033.
Key Points
- The North America RNA therapy clinical trials market size reached USD 1.01 billion in 2023 and is expected to attain around USD 1.49 billion by 2033, poised to grow at a CAGR of 3.96% between 2024 and 2033.
- North America dominated the market with the largest revenue share of 37% in 2023.
- Asia Pacific is expected to expand at the fastest CAGR of 4.52% during the period studied.
- By therapeutic areas, the rare diseases segment has held a major revenue share of 22% in 2023.
- By therapeutic areas, the anticancer segment is expected to grow at the fastest rate during the forecast period.
- By modality, the messenger RNA segment has contributed more than 37% of revenue share in 2023.
- By modality, the RNA interference segment is expected to grow at a significant CAGR in the market during the forecast period.
- By phase, in 2023, the phase II segment has held a major revenue share of 43% in 2023.
- By phase the phase I segment is expected to grow substantially during the forecast period.
The Microwave Devices Market encompasses a wide range of technologies and applications, including microwave amplifiers, oscillators, passive components, and more. These devices are crucial in telecommunications, aerospace and defense, healthcare, and consumer electronics industries, among others. The market is driven by the increasing demand for high-speed data transmission, advancements in radar and satellite communication technologies, and the growing adoption of microwave devices in various industrial applications.
Get a Sample: https://www.precedenceresearch.com/sample/4557
Growth Factors:
Key growth factors for the Microwave Devices Market include advancements in semiconductor technology, which have led to improved efficiency and performance of microwave devices. Additionally, the expanding use of wireless communication technologies, such as 5G networks, is driving the demand for microwave components that support higher data rates and bandwidths.
Region Insights:
Geographically, North America and Asia Pacific are prominent regions in the Microwave Devices Market. North America benefits from strong investments in defense and aerospace sectors, while Asia Pacific experiences robust growth due to increasing telecommunications infrastructure development and consumer electronics manufacturing.
RNA Therapy Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 3.97 Billion |
| Market Size in 2023 | USD 2.72 Billion |
| Market Size in 2024 | USD 2.82 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 3.86% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Modality, Clinical Trials Phase, Therapeutic Areas, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
RNA Therapy Clinical Trials Market Dynamics
Drivers: Drivers of the market include the rising need for efficient and reliable communication systems, the proliferation of smart devices, and the integration of microwave technology in emerging applications like autonomous vehicles and IoT devices.
Opportunities: Opportunities in the Microwave Devices Market include the development of next-generation microwave components with enhanced power efficiency and miniaturization capabilities. There is also potential in expanding applications in healthcare for medical imaging and treatment, as well as in industrial automation for sensing and control systems.
Challenges: Challenges facing the market include the complexity of designing and manufacturing high-frequency microwave components, stringent regulatory requirements, and the need for continuous innovation to meet evolving technological demands and market expectations.
Read Also: Microwave Devices Market Size to Surpass USD 14.13 Bn by 2033
RNA Therapy Clinical Trials Market Companies
- IQVIA
- ICON Plc.
- Laboratory Corporation of America Holdings
- Charles River Laboratories International, Inc.
- PAREXEL International Corp.
- Syneos Health
- Medpace Holdings, Inc.
- PPD Inc.
- Novotech
- Veristat, LLC.
Recent Developments
- In June 2023, Charles River Laboratories International, Inc. and Curigin established an alliance to manufacture adenoviral vectors. The gene therapy company will rely on Charles River’s market-leading experience in contract development and manufacturing organization (CDMO) solutions to support its preclinical and clinical studies.
- In June 2023, Moderna got the FDA’s green light for its mRNA-1273 vaccine. At the same time, Pfizer was also granted approval for their BNT162b2 vaccine. These vaccines were designed against COVID-19 for children aged from six months to five years old.
- In March 2023, Moderna submitted an IND application to the FDA regarding their mRNA-1273 vaccine against respiratory syncytial virus (RSV). This vaccine has helped reduce the possibility of respiratory tract infections in young children and infants.
Segments Covered in the Report
By Modality
- RNA Interference
- Antisense Therapy
- Messenger RNA
- Oligonucleotide, Non-antisense, Non-RNAi
By Clinical Trials Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Therapeutic Areas
- Rare Diseases
- Anti-infective
- Anticancer
- Neurological
- Alimentary/Metabolic
- Musculoskeletal
- Cardiovascular Respiratory
- Sensory
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
