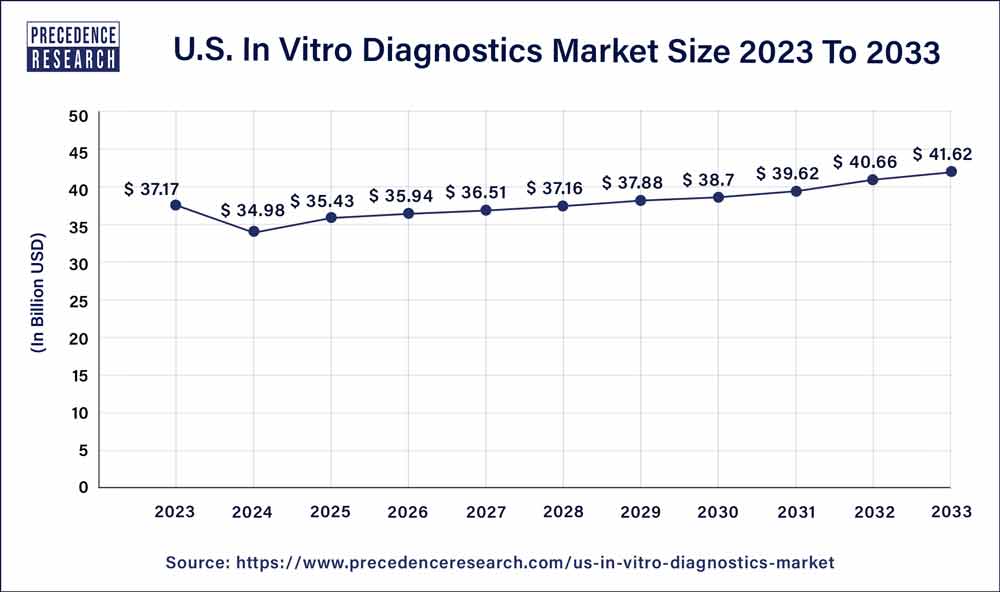
The U.S. in vitro diagnostics market size is anticipated to reach around USD 41.62 billion by 2033 from USD 37.17 billion in 2023, poised to grow at a CAGR of 2% from 2024 to 2033.
Key Takeaways
- By product, the reagents segment dominated the market with the largest share of 67% in 2023.
- By test location, the point-of-care segment dominated the market in 2023.
- By technology, the Immunoassay segment is expected to dominate the market during the forecast period.
- By application, the infectious diseases segment led the market with the largest share in 2023.
- By application, the oncology segment is expected witness the fastest rate of growth during the forecast period of 2024-2033.
- By end user, the hospital segment held the dominating share of the market in 2023.
Introduction
The U.S. in vitro diagnostics market is a dynamic sector within the broader healthcare industry, playing a critical role in disease detection, monitoring, and management. In vitro diagnostics (IVD) encompass a range of tests performed on biological samples such as blood, urine, and tissue outside the body to diagnose diseases and conditions. With advancements in technology and increasing prevalence of chronic diseases, the demand for accurate and timely diagnostic solutions continues to grow. This market segment comprises various products and services, including reagents, instruments, and software, serving diverse healthcare settings from hospitals and clinics to laboratories and point-of-care facilities.
Get a Sample: https://www.precedenceresearch.com/sample/3712
Growth Factors:
Several factors contribute to the growth of the U.S. in vitro diagnostics market. Technological advancements, such as the development of innovative biomarkers, high-throughput screening methods, and molecular diagnostic techniques, enhance the accuracy and efficiency of diagnostic tests. Additionally, the rising prevalence of chronic and infectious diseases, coupled with an aging population, drives the demand for diagnostic procedures for early detection and disease management. Moreover, the increasing adoption of personalized medicine approaches and the shift towards decentralized testing facilities further fuel market expansion.
U.S. In Vitro Diagnostics Market Scope
| Report Coverage | Details |
| U.S. Market Size in 2023 | USD 37.17 Billion |
| U.S. Market Size by 2033 | USD 41.62 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 2% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product, By Test Location, By Technology, By Application, and By End User |
U.S. In Vitro Diagnostics Market Dynamics
Drivers:
The U.S. in vitro diagnostics market is propelled by various drivers, including the growing emphasis on preventive healthcare measures and the rising awareness among patients and healthcare providers about the benefits of early diagnosis. Regulatory initiatives promoting the use of diagnostic tests for disease screening and monitoring also drive market growth. Furthermore, factors such as the increasing healthcare expenditure, favorable reimbursement policies, and investments in research and development contribute to the expansion of the market landscape. Moreover, strategic collaborations and partnerships between diagnostic companies and healthcare organizations facilitate market penetration and product innovation.
Restraints:
Despite its growth prospects, the U.S. in vitro diagnostics market faces certain restraints and challenges. These include stringent regulatory requirements for product approval and market entry, which can increase the time and cost involved in bringing new diagnostic tests to market. Additionally, concerns regarding the accuracy and reliability of certain diagnostic assays, particularly in the context of complex diseases, may impede market growth. Economic factors such as budget constraints in healthcare spending and pricing pressures on diagnostic products also pose challenges to market expansion.
Opportunities:
Despite the challenges, the U.S. in vitro diagnostics market presents significant opportunities for growth and innovation. Advances in technology, such as point-of-care testing devices and digital health solutions, open up new avenues for diagnostic companies to cater to evolving healthcare needs. Expansion into emerging markets and the development of novel diagnostic platforms for niche applications present additional growth opportunities. Moreover, the increasing focus on value-based care and patient-centered approaches creates opportunities for diagnostic companies to offer integrated solutions that improve clinical outcomes and healthcare efficiency. Strategic investments in research and development, along with a focus on regulatory compliance and quality assurance, can position companies to capitalize on these opportunities and drive sustainable growth in the U.S. in vitro diagnostics market.
Read Also: Road Rollers Market Size to Worth Around USD 6.97 Bn By 2033
By Product:
This categorization involves the various types of diagnostic products offered in the market. It includes instruments, reagents, and consumables used for conducting diagnostic tests. Products range from simple handheld devices for point-of-care testing to complex laboratory-based analyzers for high-throughput testing.
By Test Location:
This segmentation distinguishes between different settings where diagnostic tests are conducted. It encompasses point-of-care testing, which is performed near the patient at clinics, hospitals, or even at home for rapid results. In contrast, laboratory-based testing refers to diagnostic tests conducted in centralized or reference laboratories, where samples are collected and sent for analysis.
By Technology:
The IVD market utilizes various technologies to perform diagnostic tests. This categorization includes immunoassays, molecular diagnostics (such as PCR and sequencing), clinical chemistry, hematology, microbiology, and others. Each technology offers distinct advantages in terms of sensitivity, specificity, and throughput.
By Application:
Diagnostic tests serve multiple purposes in healthcare. This segmentation categorizes tests based on their intended applications, such as infectious disease testing, oncology (cancer diagnostics), diabetes management, cardiovascular disease risk assessment, autoimmune disease diagnostics, and others.
By End User:
The end-user segment classifies the types of healthcare facilities or organizations that utilize IVD products. This includes hospitals, clinics, diagnostic laboratories, research institutions, academic centers, and pharmaceutical companies. Each end user may have specific requirements and preferences for diagnostic products based on their operational needs and patient populations.
Recent Developments
- In November 2023, to create molecular tests for decentralized in vitro diagnostic (IVD) applications, Illumina, a leader in DNA sequencing and array-based technologies, and Veracyte, a top genomic diagnostics business, have partnered for several years. The partnership is centered on using Illumina’s NextSeq 550Dx equipment to develop Veracyte’s Percepta Nasal Swab test and Prosigna Breast Cancer Assay.
- In March 2023, Eli Lilly & Company and Roche announced their partnership to promote the development of Roche’s Elecsys Amyloid Plasma Panel (EAPP). A novel blood test called the EAPP promises to help diagnose Alzheimer’s disease early.
- In April 2023, to enhance health outcomes worldwide, Oxford Nanopore Technologies and bioMérieux SA, a pioneer in the in vitro diagnostics industry, announced that they have joined forces to investigate specific prospects to introduce nanopore sequencing to the infectious disease diagnostics market.
U.S. In Vitro Diagnostics Market Companies
- Alere, Inc.
- Beckman Coulter
- BD
- Bio-Rad laboratories
- Danaher
- Abbott Laboratories
- Thermo Fisher Scientific Inc.
- bioMérieux, Inc
- Quest Diagnostics
- Illumina, Inc.
Segments Covered in the Report
By Product
- Reagents
- Instruments
- Services
By Test Location
- Point of Care
- Home Care
- Others
By Technology
- Immunoassay
- Instruments
- Reagents
- Services
- Hematology
- Instruments
- Reagents
- Services
- Clinical Chemistry
- Instruments
- Reagents
- Services
- Molecular Diagnostics
- Instruments
- Reagents
- Services
- Coagulation
- Instruments
- Reagents
- Services
- Microbiology
- Instruments
- Reagents
- Services
- Others
- Instruments
- Reagents
- Services
By Application
- Diabetes
- Cardiology
- Nephrology
- Infectious Disease
- Oncology
- Drug Testing
- Autoimmune Diseases
- Others
By End User
- Standalone Laboratories
- Hospitals
- Academic & Medical Schools
- Point-of-Care
- Others
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
