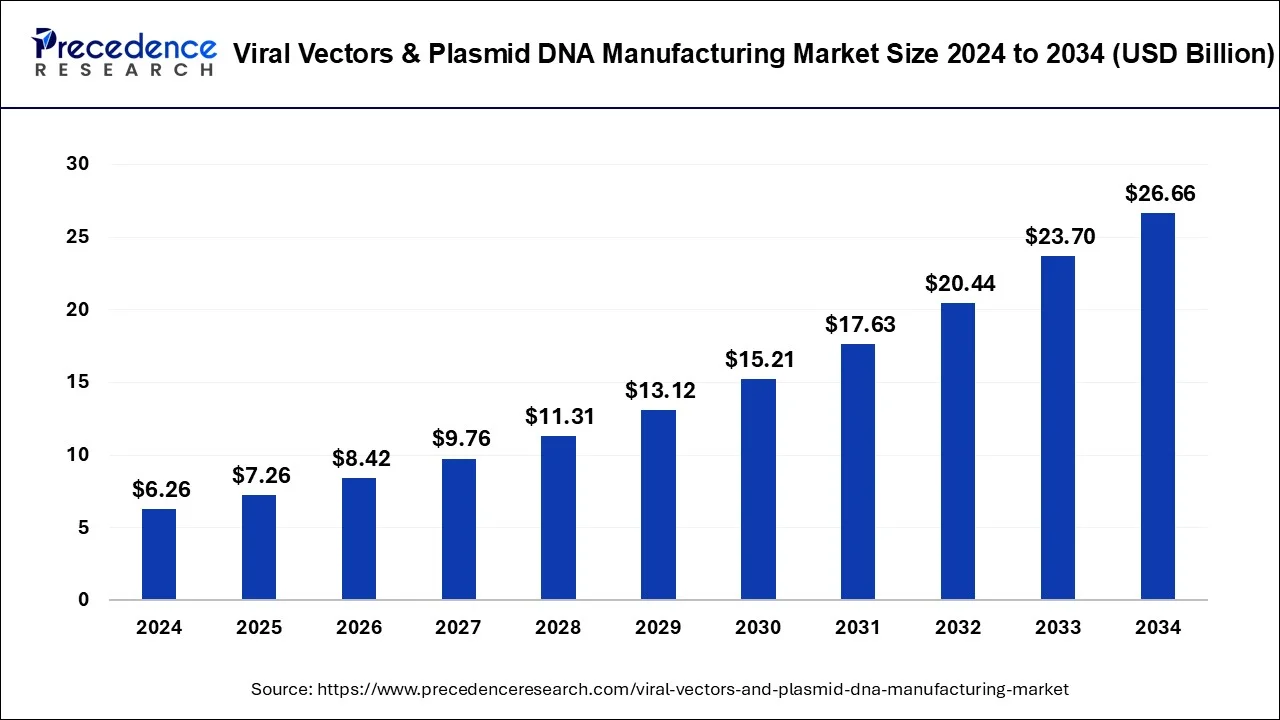
Viral vectors & plasmid DNA market to soar to USD 26.66 billion by 2034 from USD 6.26 billion in 2024, growing at a 15.59% CAGR.
Critical Observations
- North America dominated the market with a 49% revenue share in 2024.
- The AAV vector type showed strong growth, accounting for 21% of the revenue share in 2024.
- Downstream processing held a dominant position in the market with a 54% revenue share in 2023.
- Vaccinology emerged as the leading application, contributing around 22.5% of the market’s revenue in 2024.
- Cancer led the disease segment, accounting for 38% of the revenue share in 2024.
- Research institutes captured the largest revenue share, making up 58.4% of the market in 2024.
Market Scope
| Report Highlights | Details |
| Market Size by 2034 | USD 26.66 billion |
| Market Size in 2024 | USD 6.26 billion |
| Growth Rate | CAGR of 15.59% From 2025 to 2034 |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Vector Type, Application, Workflow, End-User, Disease |
| Regional Scope | Asia Pacific, North America, Europe, Latin America, Middle East and Africa |
Core Factors
The viral vectors & plasmid DNA manufacturing market is primarily driven by the rising demand for gene therapies and vaccines, advancements in gene editing technologies, and growing investments in biological drug development. The expansion of healthcare infrastructure and the increasing incidence of genetic disorders are also fueling market growth. Furthermore, more efficient production methods and enhanced regulatory frameworks are contributing to the market’s rapid expansion. AAV vector types and downstream processing continue to dominate in terms of revenue generation.
Potentials
- Increasing Demand for Gene Therapies: The growing focus on gene-based treatments, including gene editing and cell therapies, presents substantial opportunities for market expansion.
- Advancements in Vaccine Development: The rise in demand for vaccines, particularly for emerging diseases and genetic disorders, fuels the need for efficient manufacturing processes.
- Rising Healthcare Investments: Ongoing investments in biopharmaceuticals and research institutions provide a solid foundation for long-term growth in the viral vector and plasmid DNA space.
- Global Health Crises: Ongoing and future health challenges, such as pandemics and genetic conditions, will drive demand for cutting-edge gene therapies, boosting market potential.
- Regulatory Support and Innovation: More favorable regulatory environments and innovations in vector production will streamline the manufacturing process and create new growth opportunities.
Obstacles
- High Production Costs: The complex and expensive manufacturing processes for viral vectors and plasmid DNA can limit accessibility and affordability.
- Regulatory Challenges: Stringent and evolving regulatory requirements for gene therapies and biologics can slow down product development and market entry.
- Supply Chain Issues: Limited availability of raw materials and specialized equipment can cause disruptions in the production process.
- Scalability Concerns: Scaling up production to meet growing demand while maintaining quality and consistency remains a significant challenge for manufacturers.
- Ethical and Safety Concerns: The use of gene editing technologies and viral vectors raises ethical questions and safety risks, potentially limiting widespread adoption in certain regions.
Regional Insights
Currently, the U.S. is the largest market in the viral vectors & plasmid DNA manufacturing market, forecast to climb from 2024 USD 2.45 billion to 2034 USD 10.45 billion. Growth is also spurred by FDA approvals for advanced therapies and significant investments in gene therapy manufacturing. Clinically, it leads in North America, where 68% of studies occurred. Europe is also a big market, with 21% of trials. Multinational collaborations to enhance the production process in Asia Pacific is emerging as the next gene therapy manufacturing hub.
Don’t Miss Out: Surgical Robotics Market
Industry Leaders
- Novasep
- Aldevron
- MerckWaismanBiomanufacturing
- Creative Biogene
- The Cell and Gene Therapy Catapult
Recent News
2024 marks a transformative year for cell and gene therapy (CGT) innovations. The global introduction of Amtagvi, a Tumor-Infiltrating Lymphocyte (TIL) therapy, signals a new frontier in solid tumor treatment. The approval of PM359 for clinical trials marks a significant milestone in gene editing, while INT2104, the first in vivo modified CAR-T therapy, is poised to broaden CAR-T treatment possibilities. Additionally, the A-T Children’s Project will host an international conference in November, focusing on gene therapy advancements for ataxia-telangiectasia (A-T), bringing together industry and academic leaders.
Market Segmentation
By Vector Type
- Adenovirus
- Plasmid DNA
- Lentivirus
- Retrovirus
- AAV
- Others
By Application
- Gene Therapy
- Antisense &RNAi
- Cell Therapy
- Vaccinology
By Workflow
- Upstream Processing
- Vector Recovery/Harvesting
- Vector Amplification & Expansion
- Downstream Processing
- Fill-finish
- Purification
By End-User
- Biopharmaceutical and Pharmaceutical Companies
- Research Institutes
By Disease
- Genetic Disorders
- Cancer
- Infectious Diseases
- Others
